The Universal Mining Machine
Posted by Ugo Bardi on January 24, 2008 - 10:49am in The Oil Drum: Europe

The coal mine of Garzweiler, Germany, in a Google Earth image. The satellite has caught two giant mining machines at work. Measured with the google ruler, each "arm" of the machines measures about 120 m (ca. 400 ft). These are not universal mining machines, but give some idea of the scale of modern mining operations. The Garzweiler mine is said to hold more than a billion tons of coal reserves.
Introduction
In a science fiction story that I had in my hands, many years ago, a group of explores stranded on a remote planet needed to build a new spaceship using local materials. They had no time and no resources for traditional mining, so they built a "universal mining machine" that extracted elements from the planet's crust. The machine crushed rock, heated it and transformed it into an atomic plasma. The ions in the plasma were accelerated and then separated according to mass by a magnetic field. In input, you had just ordinary rock; at the output, you had all the elements present in the original rock, each neatly packed in its own box.
That story (I think it was by Poul Anderson) has always fascinated me. Why can’t we build a machine like that here, on Earth, and stop worrying about running out of mineral resources? Some economists seem to think of depletion in these terms, indeed; as if they really had a universal mining machine ready. A favorite statement that you can hear on this subject is that there is no such thing as finite resources. Prices create resources depending on what you need. If prices are high enough, you can always make a profit even extracting from a very low grade ore. Since you can't run out of crust to mine, you'll never run out of anything or, at least, you'll see no problems for a long, long time. Julian Simon, author of the successful book “The Ultimate Resource” (1985), was perhaps the champion of this school of thought. Among other things, he said that we have mineral resources for “7 billions of years” (Simon 1995).
Recently, this kind of enthusiasm about the abundance of resources seems to have become less popular. However, the general opinion is still one of optimism, as shown in innumerable articles in the mainstream press whenever the question of depletion is taken up. Unfortunately, there are problems with the idea that non-physical entities, prices, can create physical entities, mineral resources. Prices are just tags, labels that you stick on something. If you need to extract and process something, it is not enough to change the label on it: you need energy.
Energy is the physical entity that defines what you can extract and what you can't. In a way, Simon and his followers are right in saying that the amount of mineral resources is not fixed. But the amount of extractable resources is defined not by prices but by the amount of energy you can afford to employ for extraction. And, unlike prices, energy is a limited resource.
In the future, the energy supply it may well go down as fossil fuels are gradually depleted. If we consider also the problem of the progressive depletion of high grade ores, it is clear that the extractive industry faces a a formidable challenge. How long can we keep on mining at the present levels? Will we be able to keep the industrial society working? Is there anything like "sustainable mining?"
These questions are difficult to answer but can’t be ignored any longer. Several recently published papers emphasize the finiteness of the resources and the likely problems that we will be soon facing (Gordon et al (2006), Ayres (2007), Pickard (2007), Cohen (2007)). In a recent work published on “The Oil Drum” Ugo Bardi and Marco Pagani (2007) have shown that the mineral production of several metals and compounds has peaked and is now declining. Other metal commodities show signs of impending peaks. All that, of course, is evidenced also by the increasing price trends of all mineral commodities in the past few years. Clearly, we are not talking of something far away in the future but of something that may be starting to occur right now.
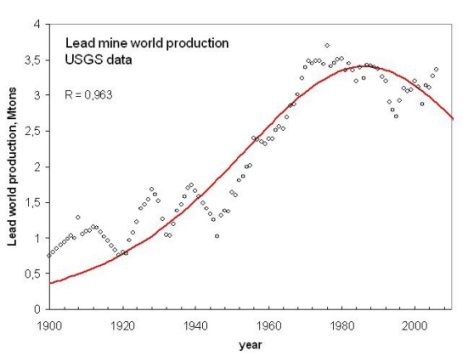
An example of the "bell shaped" production curve of some mineral resources, in this case, lead. From Bardi and Pagani 2007
Earth's mineral resources
The Earth's crust is said to contain 88 elements in concentrations that spread over at least seven orders of magnitude. Some elements are defined as “common,” with concentrations over 0.1% in weight. Of these, five are technologically important in metallic form: iron, aluminium, magnesium, silicon, and titanium. All the other metals exist in lower concentrations, sometimes much lower. Most metals of technological importance are defined as "rare" and exist mostly as low concentration substituents in ordinary rock, that is, dispersed at the atomic level in silicates and other oxides. The average crustal abundance of rare elements, such as copper, zinc, lead and others, is below 0.01% (100 ppm). Some, such as gold, platinum and rhodium, are very rare and exist in the crust as a few parts per billion or even less. However, most rare elements also form specific chemical compounds that can be found at relatively high concentrations in regions called "deposits". Those deposits from which we actually extract minerals are called "ores".
The total amount of mineral deposits in the crust is often described as inversely proportional to grade ("Lasky's law"). That is, low grade deposits are much more common than high grade ones and contain a much larger amount of materials. As a consequence, when the progressive depletions of high grade ores forces the mining industry to move to low grade ores, you have the counterintuitive effect that the amount of resources available increases ("you don't run out of resources, you run into them", as Odell said in 1994). This apparent abundance is one of the reasons for the great optimism of some people about the availability of minerals. Unfortunately, this abundance is an illusion for several reasons; one is that Lasky's law is not valid for the whole range of crustal concentration.
According to Brian Skinner (1976, 1979), the amount of a resource in Earth's crust is not simply inversely proportional to concentration. Rather, the distribution is "bimodal", that is there is one large peak for the element as a low concentration substituent and a smaller peak for the same element in deposits. The absence of concentrations in between the two peaks is what Skinner terms the "Mineralogical Barrier." The concept is shown in the following figure.

The concept of Skinner's "mineralogical barrier". The relative size of the two peaks is not to scale.
We don't have enough data for building Skinner's diagram to scale but, for a rough estimation of the different size of the two peaks, we can make a quick calculation for the case of copper. For the low concentration peak, we can consider that the average amount of copper in the upper crust is reported to be around 25 parts per million (Wikipedia 2007). Considering a land area of 150 million km2, and an average rock density of 2.6 g/cc, we can calculate something like ten trillion tons of copper available within one km depth from the surface. For the high concentration peak, we lack complete data, but we can consider that the USGS estimates the copper global land based resources as ca. 3 billion tons. The actual size of all the existing copper deposits is surely larger, but it should be not far from this order of magnitude. Therefore, the ratio in size of the two peaks is of at least of a factor one thousand.
There are exceptions to Skinner's model, uranium for instance seems not to have a double peak (Deffeyes 2005) and that may be related to the specific chemical characteristics of uranium ions. Then, of course, the common minerals, iron for instance, exist in high concentrations all over the crust and don't have a real mineralogical barrier. But the bimodal distribution is probably the general condition of practically all the rare metals.
Mining
Mining is a multi-stage process. The first is the extraction phase, in which ore materials are extracted from the ground. Then, there follows the beneficiation stage, where the useful minerals are separated from the waste (also called "gangue"). Further processing stages normally follow; for instance the production of metals requires a reduction stage and a refining one. All these stages require energy. To be exact, we should rather use the concept of "exergy" instead of energy, but in the context of mining the difference is marginal.
Let's make a practical example. Today, we extract copper from ores - mainly chalcopyrite, CuFeS2 - that contain it in concentrations of around 1%-2%. The energy involved in the extraction, processing and refining of copper metal is in the range 30-65 megajoules (MJ) per kilogram (Norgate 2007) with an average value of 50 MJ reported by Ayres (2007). Using the value of 50 MJ, we need about 0,75 exajoules (EJ) for the world’s copper production (15 million tons per year). This is about 0.2% of the world total yearly production of primary energy (400-450 EJ) (Lightfoot 2007).
The following table lists the specific energy needed for the production of some common metals, together with the total energy requirement for the present world production
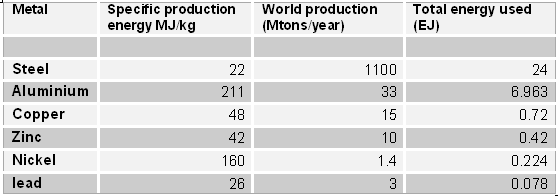
Specific and total energy of production for some metals. The data on the specific energy are from Norgate and Rankin (2002). Those on production are from the United States Geological Survey (USGS) for 2005.
Note how the world's production of steel alone requires an amount of energy (24 EJ) equivalent to about 5% of the total of the world's supply (ca. 450 EJ). Since making steel requires coal, this datum is in approximate agreement with the fact that 13% of the world's coal production goes for steel and that coal accounts for about 25% of the world's primary energy (source www.worldcoal.org).
Taken together, these data indicate that the total energy used by the mining and metal producing industry might be of the order of 10% of the total. This estimation seems to be consistent with that of Rabago et al (2001) who report a 4%-7% range and those of Goeller and Weinberg (1978) of 8.5% for the metal industry in the United States only.
Facing the mineralogical barrier
Over the history of mining, we have extracted minerals from high grade ores exploiting the energy provided for free by geochemical processes of the remote past (see De Wit 2005). Ores embed a lot of energy, either generated by the heat of Earth's core or by solar energy in combination with biological processes. The Earth is a geochemically live planet and the existence of ores and deposits is a consequence of that. But the processes that created ores are extremely rare and ores are a finite resource.
There is little hope to find high grade sources of minerals other than those we know already. The planet's crust has been thoroughly explored and digging deeper is not likely to help, since ores form mainly because of geochemical (especially hydrothermal) processes that operate near the surface. The oceans' bottom could be a source of minerals (Roma 2003) but so far not a single gram of anything has been extracted from there. The oceans themselves contain metal ions but in extremely minute concentrations. With the possible exception of uranium (Seko 2003), extracting minerals from seawater is out of question. For instance, all the copper dissolved in the oceans would last for just ten years of the present mine production (Sadiq 1992). Finally, there is the old science fiction of dream of mining the Moon and the asteroids. But, if our problem is energy, then we can't afford the energy cost of traveling there. Besides, the Moon and the asteroids are geochemically "dead" and contain no ores.
Therefore, as we keep mining, we have no choice but to move down progressively towards to low grade ores. In general, the energy required for extracting something from an ore is inversely proportional to the ore grade. That is, it takes ten times more energy to process an ore which contains the useful mineral in a ten times lower concentration (Skinner 1979). This relation holds for ores of the same composition which just change in grade.
We may also completely run out of a certain kind of ore and have to switch to ores of different chemical composition. That has already happened in the past, for instance for native metals. Iron, for instance, was once found in metallic form, ready to be forged, in the form of meteorites. That source has been completely exhausted as a mineral resource long ago. Switching ore normally involves an upward step in the amount of energy required.
Depending on the kind of product, the change in ore grade may have a large effect, or almost none, on the total energy requirement. Aluminium, for instance, is an extreme case in which extraction and beneficiation play a minor role (Norgate and Rankin, 2000). This is not surprising, considering that we extract aluminium from bauxite ores which contain it in a high concentration, about 30%-70% as aluminium oxide. The situation is different from most other metals, where ores usually contain a much smaller fraction of the useful element. Gold is an example in which almost the whole energy requirement is in extraction and beneficiation. Copper is an example of an intermediate situation where about 50% of the energy goes for extraction and beneficiation.
The depletion of high grade ores is a problem that, eventually, will lead us to face Skinner's mineralogical barrier. The amount of minerals on the "other side" of the barrier is huge. If we could manage to extract from this region of concentrations, we wouldn't have problems of depletion forever or at least for the "7 billion years" that Julian Simon mentioned. However, that would require an amount of energy well beyond our present capabilities.
Let's make an approximate calculation for evaluating this energy. Consider copper, again, as an example. Copper is present at concentrations of about 25 ppm in the upper crust (Wikipedia 2007). To extract copper from the undifferentiated crust, we would need to break down rock at the atomic level providing an amount of energy comparable to the energy of formation of the rock. On the average, we can take it as something of the order of 10 MJ/kg. From these data, we can estimate about 400 GJ/kg for the energy of extraction. Now, if we wanted to keep producing 15 million tons of copper per year, as we do nowadays, by extracting it from common rock, this calculation says that we would have to spend 20 times the current worldwide production of primary energy. Prices can't make common rock a source of rare metals any more than ghost shirts could make Indians invulnerable to bullets.
Of course, this is just a rough order of magnitude estimation. We may not need to really pulverize the rock at the atomic level and we may find areas of the crust which contain more copper than average. For instance, Skinner (1979) proposed that we could extract copper from a kind of clay named biotite and that would need a specific energy of extraction approximately ten times larger than the present requirements. If the problem were copper alone, that would be doable. But if we have to raise the energy requirement of a factor of ten for all the rare metals, clearly we rapidly run into levels that we cannot afford, at least at present.
The future of mining
In the short run, we don't seem to face critical problems in terms of ore supply, at least as long as we can keep our energy supply stable. Let's consider copper again as an example. The U.S. Geological Survey (USGS) estimates the world copper reserve base at 950 million tons (2007) (although Grassmann and Meyer (2003) report a lower value). If we could keep a steady extraction rate, we would have around 60 years of copper supply. Of course, the extraction rate has never been constant over the extractive history of copper. A more realistic model (Bardi and Pagani 2007) takes into account the growth and decline of the supply and sees the copper production peak in about 30 years from now.
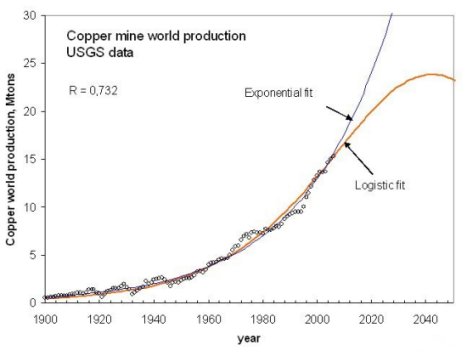
a projection of the production rate of copper metal from mining. From Bardi and Pagani, 2007
30 years to peaking, or 60 years to total exhaustion, may look uncomfortably close, but it is not tomorrow. In many other cases, we don't seem to be close to total exhaustion (e.g. Cohen 2007). However, there are cases where depletion looks like a more pressing problem, such as for indium, a metal important for the electronics industry and that may be in short supply soon. Also, some metals may be facing serious depletion problems because of an increase in the demand. For instance, if we were to use fuel cells on a large scale for road transportation, the known reserves of platinum would be most likely insufficient for the catalytic electrodes. (Department of Transport 2007)
These are serious problems, but are marginal in comparison to the real problem we have, which is also much more immediate. Ores, as we said, are defined in terms of the energy necessary for exploitation. To keep mining from the present ore supply, we need at least a constant supply of energy. But, in the near future, our energy supply may go down instead of up. Dwindling energy supply affects all the stages of production of mineral commodities, not just the extraction and beneficiation. That can have immediate and adverse effects on the production of mineral commodities.
Today, the energy used in extracting and processing minerals comes mainly from fossil fuels and, in some cases, it is directly dependent on liquid fuels produced from crude oil. For instance, it is reported (DOE 2007) that 34% of the energy involved in the US mining industry is in the form of diesel fuel. Fossil fuels are a mineral resource that has been heavily exploited in the past and they are undergoing rapid depletion and are expected to peak within a few decades at most. Peaking in the production of a mineral resource is a general phenomenon which is related to the increasing costs of prospection, extraction and processing as the resource becomes rare and more expensive. At present, crude oil is approaching its worldwide production peak ("peak oil") and is expected to start an irreversible productive decline in the coming years (see e.g. www.peakoil.net). The other two main fossil fuels, natural gas and coal, are expected to peak at a later time, but in the coming decades anyway.
We don't need to wait for the actual production peak to see a resource becoming more expensive both in terms of energy and in monetary terms. If it takes more energy to extract and refine oil, this extra investment in energy will directly affect the extraction processes that make use of oil as an energy source. So, if the present trend of decline in the production of fossil fuels continues, we won't be able to exploit all the mineral resources that exist on the "good" side of the mineralogical barrier. If nothing changes, in a not far future we are going to see a decline in the production of all mineral commodities: "peak minerals" (See Bardi and Pagani 2007). Peaking of minerals production poses a serious and immediate problem in terms of maintaining a supply of mineral commodities to the world's economy.
Mitigation strategies
How to react to the future decline of minerals production? There are two ways: either we stimulate or force mines to produce more, or we use more efficiently what we can still manage to produce. We can list a few more detailed strategies: 1) crossing the mineralogical barrier, 2) substituting, 3) recycling, 4) reusing, and 5) doing with less.
1. Crossing the mineralogical barrier. This strategy is equivalent to building - and fueling - a real universal mining machine and extract the minerals we need from the undifferentiated crust. That would solve the problem once for all and Julian Simon's dream (resources for 7 billion years) would become true. This kind of mining would be "sustainable", in the sense that it could last as long as we could provide the large amount of energy needed for it. The planet's surface would not look pretty after the passage of these giant lumbering monsters but, if we had enough energy for fueling them, we could probably afford to move the whole operation to space. Probably, nobody would complain for the ruined aesthetics of remote asteroids. However, as we saw, the energy requirements for such a technology are way above anything we can conceive for the near future. It would require a radical technological breakthrough in energy production, perhaps a new form of nuclear fusion technology. We can't dismiss this possibility, but we cannot count on it.
2. Substitution. Already in 1976, Brian Skinner had titled one of his papers “A second age of iron?”. He he meant that the future could see a general shift of industrial processes away from rare elements, towards common ones, such as iron. In the same year (1976), Goeller and Weinberg had examined the situation in a paper in which they had proposed what they had called "The principle of infinite substitutability." Their work is cited sometimes as the ultimate demolition of catastrophism. But they had correctly recognized that substitution requires profound changes in technology and society. Something that both Skinner and Goeller&Weinberg had failed to state explicitly was that substitution requires energy, and often a lot of it.
Let's make a few examples of substitution in order to illustrate the energy problem. A classic one is that of replacing copper with aluminium as a conductive material. Aluminium is one of the common metals in the crust and using it in place of copper looks promising against the problem of ore depletion. Aluminum is a poorer conductor and it is flammable when it overheats but, with some precautions, it is possible to use it for almost all power carrying operations. The problem is that, as we saw, it takes 210 MJ/kg to produce metallic aluminum whereas it takes only around 50 MJ/kg to produce one kg of copper. Since our most pressing problem is energy, not ore grade, the idea of substituting copper with aluminum is a solution for the wrong problem.
Let's make another example. Suppose we have problems with chromium's availability. In this case, we are in trouble with the production of stainless steel, which contains chromium in relatively large amounts. For many structural applications that require strength and resistance to corrosion, stainless steel could be substituted with titanium (Goeller and Weinberg 1976). Unfortunately, titanium is a high melting point metal which requires large amounts of energy for its production. According to Norgate et al. (2007) we need 361 MJ/kg for the production of titanium metal, against just 75 MJ/kg for stainless steel. Again, the substitution strategy turns out to be power hungry.
A further example is mercury. Goeller and Weimberg take mercury as their paradigm of substitutability, since it has been nearly completely phased out from technological uses during the past decades. But it is also true that this substitution has required energy. We have no data for the energy needed to produce a kg of mercury, but probably it was not very large. In most cases, the substitutes required most likely more energy. Consider, for instance, that mercury in vacuum pumps has been substituted by synthetic oil that are manufactured from precursors made from crude oil. This kind of substitution required energy for the synthesis of the oil, as well as for the periodic replacement of the fluid that lasts less than mercury. This, and other kinds of substitution can hardly be defined as steps towards sustainability.
So, substitution is a strategy that can counteract ore depletion but at a high price in terms of energy. It is not as energy hungry as the universal mining machine, but the "universal substitution" of all the rare metals would require more energy than what we can reasonably assume to have in the near and medium term future.
3. Recycling . If we could recycle at 100% efficiency, we would never run out of anything. However, the problem is the same as with conventional mining: recycling takes energy. It doesn’t need the huge amounts of energy that would be necessary for mining undifferentiated rock, nevertheless high efficiency recycling turns out to be very difficult for several reasons.
Managing wastes seems to be a typical example of our tendency of discounting the future at high rates (Hagens 2007). Waste is considered a nuisance rather than a stock of resources. If we don’t find that it is convenient to recycle something, we just dump it in a landfill or we burn it in an incinerator. In both cases, the result is that post-recovery is nearly impossible. In the case of incinerators, the finely dispersed ashes produced are a mix that would require extremely complex and expensive treatments in order to recover specific metals (Shen and Fossberg, 2003) and, at present, it is not done. For landfills, recovery might be easier, but still we dump together valuable metals and potentially toxic waste and that doesn't make recovery easy. At present, landfills are not exploited as sources of minerals at the industrial level although it is reported to be done in third world countries. That is possible, however, only at a high cost in terms of health hazards for of those engaged in the task.
The result is that we manage to recover only a fraction of what we throw away. According to the USGS (Papp 2005), in the United States the average recycling rate is of about 50% in weight for the principal metals produced. The maximum recycling rate is of 74% in the case of lead. Iron is recycled at about 50%; other common metals do less well: both copper and aluminium are not recycled at more than about 30%. Norgate and Rankin (2002) report different values, but the average level of recycling for most common metals remains of the order of 50%.
This is not enough for compensating the decline of mining. If we recycle something at 50% it means that after just 4 cycles of recovery we have lost more than 90% of the material we started with. We would need to do much better than that but, evidently, it is not easy and it would take a radical change in the way industrial production is conceived and managed. That, in turn, would require a degree of centralized planning that is unlikely to materialize until the shortage of materials becomes very serious. Again, it is our tendency of discounting the future at high rates (Hagens 2007)
4. Reuse . Re-using means to produce long lasting products that can be repaired and/or refurbished. Reusing requires some energy, but probably less than any of the other strategies we have considered so far. As an example, we can think of making car bodies in stainless steel or in titanium. The energy required for making stainless steel (Norgate 2007) is about twice that needed for ordinary steel, while titanium would require about ten times as much. However, a car made in stainless steel or titanium would never rust and would last practically forever. Of course, this kind of strategy goes against the grain of everything that is normally thought as a successful strategy in the car business. Designing products in view of reusing them has never been popular and, in general, reuse smacks of poverty; not just with cars. It hard to imagine that with our limited ability of planning ahead (Hagens 2007) we could change our attitude. However, if a crisis of energy hits us, we'll be forced to use the products we have for longer times, with all the limits and problems involved. We might also have to reuse products for purposes they were not designed for. In Southern Europe or North Africa, you can find people who can make ashtrays out of soft drink cans. It is thought as a gadget for tourists, so far, but that might change in the future.
5. Doing with less , This is the easiest strategy; one that doesn't require energy at all. Simply, if you can't afford something, you don't use it. It also doesn't require any government intervention. With less energy and less materials available, you may discover that you can't afford a SUV for everyday commuting. So, you may switch to a subcompact. Even better, you can switch to a bicycle; or you may walk. Eventually, you may be able to commute at all. There is a lot of useless fat that society can shed and still function in a way that is recognizable to us. The problem is that, while shedding this and that, society may enter a deadly downward spiral that gradually destroys the world's industrial base. The process may lead us back to where we started before the industrial revolution: to a low population agrarian society with a low energy surplus. Such a society could not maintain the technological level that we have reached.
Interestingly, our farming descendants wouldn’t need to go back to flint knapping. Recovering just a fraction of the more than 50 billion tons of iron that we have produced in the past centuries, they would have have plenty of supply for all their conceivable needs. Just think that at the time of Napoleon, when the industrial revolution had already started, the whole world production of iron and steel was less than a million tons per year, about one thousandth of what it is today. With the scraps recovered from our smelting work, our low tech descendants could go on happily forging swords and ploughs (and perhaps even muskets and cannons) for many thousands of years. The metal leftovers from our civilization would also provide them with thousands of years of supplies of other metals, at least of the kind that can be smelted or forged in a charcoal furnace. That excludes titanium and some exotic metals, but leaves all the rest. Think that our society has produced so much copper metal that an amount of about 200 kg per person is still around in the industrialized world (Gordon 2006). With so much copper, our agrarian descendants would have plenty of bronze for pots and pans and for spectacular pieces of statuary as well. They would even have aluminium; something that our pre-industrial ancestors couldn’t even dream of.
Conclusion and perspectives
Our civilization has deeply changed the chemical composition of the upper crust of the Earth. Elemental deposits that were formed in hundred of thousands of years of geochemical processing (Shen 1997) have been removed, transformed, and in large part dispersed. Hundreds of thousands of years (at least) will be needed to reform these deposits, and times at least of the same order of magnitude will be required for restocking the planet with oil and natural gas. Some minerals, such as coal, have been formed in specific conditions in the remote past and may never reform again in the future in the amounts that existed before we started extraction.
We inherit from past generations a planet that is very different from what it was before the industrial revolution. The cheap and abundant minerals that our ancestors have used to build the industrial society are no more. If we want to keep going along the industrial path, we'll need to develop new strategies to insure a sufficient supply of materials. That will depend mostly on energy. It will be our capability of producing energy that will determine the future choices of society.
If we'll succeed in increasing the energy supply, then substitution can compensate for the decline in ore grade and, if we really can manage to have plenty of energy, we may put into practice the dream of an infinite supply of minerals by mining the asteroids using a universal mining machine. However, such a scenario doesn't look very likely.
It is much more probable that, in the future, we will not be able to compensate the dwindling supply of fossil fuels with nuclear or renewable energy. This will lead to an overall reduction of the world energy supply and, coupled with the gradually depletion of high grade ores, a reduction of the availability of all mineral commodities. The reaction to this situation will be a combination of low energy strategies: recycling, reusing, and doing with less.
In the worst case hypothesis, considering also the likely damage deriving from climate change, the crisis could be so bad that it may push us back to an agrarian society. With the scraps left by our civilization, it would be a metal rich kind of agrarian society, but still a low technology one. Could it ever restart with a new industrial revolution? It is difficult to say. The industrial revolution that we know was strictly linked with the availability of cheap coal and that is gone forever after we burned it. It is hard to run Satanic mills with wood charcoal only; forests tend to run out too fast. Perhaps there will be only one industrial revolution in the history of mankind.
In between these two extremes, mining the asteroids and returning to subsistence agriculture, it is perfectly possible to imagine intermediate scenarios. We may conceive a society that keeps a supply of energy smaller, but not much smaller, than the present one and that manages to use it to keep a reduced, but non zero, supply of minerals. It would have to be extremely careful to avoid wasting materials and it would see some of our habits - air travel, for instance - as dangerous extravagances. It would have to recycle and reuse at a level that would appear difficult to conceive for us. In some ways, the attitude of that society would compare to that of the thrifty world of Japan of the Edo period (JSN 2003). Such a society could maintain our technological level and improve it. It could manage the planet's climate and, perhaps, remedy the damage that we have done to it. It can still engage in the exploration of space, in fundamental research, in the development of artificial intelligence and other cultural and human pursuits that can't be conceived without a healthy surplus of energy and materials.
If we'll ever arrive to such a society, it is difficult to say. We would need to start planning for it already now, but our capability of long term planning is very limited (Hagens 2007). At least, from this discussion, we can say that our immediate concern should not be just energy but also the availability of basic materials for industry.
Acknowledgement: the author thanks Franco Galvagno, Marco Pagani, and Antonio Tozzi for their suggestions and comments on this paper
References
Ayres, R. 2007, Ecological Economics vol. 61, p. 115 – 128
Bardi, U. and Pagani M. 2007, “Peak Minerals”, The Oil Drum, http://europe.theoildrum.com/node/3086
Cohen, D., 2007 “Earth's natural wealth: an audit”, From issue 2605 of New Scientist magazine, 23 May 2007, page 34-41. http://www.science.org.au/nova/newscientist/027ns_005.htm
Commonwealth Government Initiative, 2000, “Industry, Science and Resources Energy Efficiency Best Practice Program” http://www.industry.gov.au/assets/documents/itrinternet/aluminiumsummary...
Deffeyes, K., 2005 “Beyond Oil”, Hill and Wang ed., New York
Department of transport, 2007 (accessed) "Platinum and hydrogen for fuel cell vehicles" http://www.dft.gov.uk/pgr/roads/environment/research/cqvcf/platinumandhy...
De Wit, M. 2005, "Valuing copper mined from ore deposits"
Ecological Economics, vol. 55 pp. 437– 443
DOE, Department of Energy, 2007 "Mining Energy Bandwidth Analysis Process and Technology Scope
http://www1.eere.energy.gov/industry/mining/pdfs/mining_bandwidth.pdf
Goeller, H.E. Weinberg, (1976)"The Age of Substitutability" The American Economic Review, Vol. 68, No. 6. pp. 1-11.
Gordon R. B., Bertram M., and Graedel T. E., 2006, “Metal stocks and sustainability”, Proceedings of the national academy of sciences, PNAS, January 31, vol. 103, no. 5, pp. 1209–1214
Hagens, N. 2007, “Climate Change, Sabre Tooth Tigers and Devaluing the Future”, http://www.aspo-ireland.org/index.cfm?page=speakerArticles&rbId=8
JSN - Japan for Sustainability newsletter, 2003 http://www.japanfs.org/en/newsletter/200303-1.html
Lightfoot, H.D., 2007, "Understand the three different scales for measuring primary energy and avoid errors", Energy vol 32 pp. 1478–1483
McNulty, 1994, http://www.goldfever.com/gold_sea.htm
Meyer, F. M. & Grassmann, J. (2003) Erzmetall 56, 349–355.
Norgate T. E. and Rankin W.J., 2000 "Greenhouse gas emissions from aluminium production – a life cycle approach", CSIRO paper, www.minerals.csiro.au/sd/CSIRO_Paper_LCA_Al.htm
Norgate. T.E. 2001 "A comparative life cycle assessment of copper production processes", CSiro Minerals Reports DMR 1768
http://www.intec.com.au/docs/media/2001%20Oct-CSIRO%20Life%20Cycle%20Ana...
Norgate, T., Rankin, J., 2002 "Tops at Recycling, Metals in sustainable development", CSIRO sustainability papers, http://www.bml.csiro.au/susnetnl/netwl30E.pdf
Norgate T.E., Jahanshahi, S. Rankin W.J. 2007,"Assessing the environmental impact of metal production processes" Journal of Cleaner Production vol 15 pp 838-848
Odell, P.R., 1994, World oil resources, reserves and production. The Energy Journal (IAEE) 15 Special Issue, pp. 89–113.
Pickard, W.F., Geochemical constraints on sustainable development: Can an advanced global economy achieve long-term stability?
Global and Planetary Change (2007),
Roma, P. 2003, “Resources of the sea floor”Science 31 January 2003Vol. 299. no. 5607, pp. 673 – 674
Seko, N. Katakai, A. Hasegawa, S., Tamada, M., Kasai, N., Takeda, H., Sugo, T., Saito, K., 2003 "Aquaculture of Uranium in Seawater by a Fabric-Adsorbent Submerged System" Nuclear Energy Volume 144, Number 2 Pages 274-278
Skinner, Brian J. 1976. “A Second Iron Age Ahead?”American Scientist, vol. 64 (May-June issue), pp. 258-269.
Skinner, B. 1979, Proc. Natl. Acad. Sci. USA Vol. 76, No. 9, pp. 4212-4217
Simon, J., 1985, http://www.juliansimon.com/writings/Ultimate_Resource/
Simon, J, 1995, The State of Humanity: Steadily Improving
Cato Policy Report, http://www.cato.org/pubs/policy_report/pr-so-js.html
United States Geological Survey (USGS). Mineral commodities information, minerals.usgs.gov/minerals/pubs/commodity/
Papp, J.F. 2005 “Recycling Metals”, United States Geological report http://minerals.usgs.gov/minerals/pubs/commodity/recycle/recycmyb05.pdf
Rábago, K.R., Lovins A.B., Feiler T.E., 2001 "Energy and Sustainable Development in the Mining and Minerals Industries", IIED report, http://www.iied.org/mmsd/mmsd_pdfs/041_rabago.pdf
Sadiq, M. 1992 "Toxic Metal Chemistry in Marine Environments" Taylor & Francis, ISBN: 0824786475,
Shen, H. Fossberg, E. 2003 "An overview of recovery of metals from slags" Waste Management Volume 23, Issue 10,Pages 933-949
Stein, H.J., Cathles, L.M., 1997 “The Timing and Duration of Hydrothermal Events” Bulletin of the Society of Economic Geologists Volume 92 November-December Number 7/8 pp. 763-765, http://www.segweb.org/eg/papers/DurationPreface.htm
Wikipedia, 2007 (accessed) “Abundance of the elements” http://en.wikipedia.org/wiki/Abundances_of_the_elements_%28data_page%29



Whoa! I enjoyed that ever so much.
I still want to digest but thank you for your work.
All we need is the universal minning machine and that enzime that breaks the molecular structure of Cellulose fiber allowing production of human digestable foods and we are good to go.
P.S. sounds a little like Lindon LaRouches Isotope economy meets an Asimov novel.
Seriously thanks for your work.
Great post. Clearly energy available constrains the production of nearly everything, including metals.
A general observation - if a "universal mining machine" is ever developed, it will probably be used first to mine the Moon (or asteroids). Free access to unlimited solar power (for up to two weeks out of every month at least), and free access to a vacuum (needed to separate atoms efficiently). Lunar produced helium-3 for fusion reactors has been suggested as an alternative energy source. Agree that there are probably no ore deposits on the Moon to speak of, unless you count metallic broken pieces of meteorites, or metallic ores possibly concentrated at the base of thick lava flows. Impacts have homogenized everything else (free comminution). John Lewis (University of Arizona) has argued in a couple of books in favor of mining asteroids instead, mainly owing to the value of platinum-group elements alloyed with the native iron-nickel, the presence of water and other volatiles, and freedom from gravity constraints on transport. Very high (perhaps far too high) initial investments will be required for any type space mining operations, however, and it's unclear that anyone could make a profit on them.
Minor quibble with your statement about the sea floor: Diamonds and even nuggets of tin oxide have long been recovered from fairly shallow water at very low energy cost (many from illegal dredging operations - basically a pump at the end of a very long hose - a type of placer mining). The main constraint on sea floor mining is the huge amount of turbidity pollution produced, and the legal problem that most of the sea floor currently belongs to no single country. Metallic mineral sulfide deposits are continually being created by sea floor hot springs ("black smokers") at spreading centers (mid-ocean ridges) and are being destroyed as sea floor is subducted at trenches. Other metals are present in manganese oxide nodules at the surface. I suspect that, if the need becomes acute and industrial civilization continues its present course, despite PO, the 70% of the planet that is sea floor will be the next metal prospecting frontier - way ahead of outer space. By then little marine life may survive anyway. More likely even less would survive such a mining episode.
Another quibble. As Deffeyes (2005) discusses in his book, the 1970's assertion by Brian Skinner of a concentration valley between high-grade ores and geochemical background levels of metals has yet to be demonstrated scientifically, AFAIK, and Deffeyes makes a pretty good case that it doesn't hold for uranium in particular. Whether or not such a "valley" exists has no effect on your basic argument - that the energy costs of extraction eventually get prohibitive as ore grade goes down, implying that eventually metal production must peak unless energy continues to get cheaper OR if the metal is needed "at any price". Uranium is probably such a metal - the cost is at present a tiny fraction of the cost of building a new power plant, and could therefore increase 10X or even 100X without affecting the basic economics much, especially if fuel reprocessing was used. Indium for solar cells might be another "at almost any price" metal if no better technology is developed.
The basic problem of economists is that they think of petroleum as metal ore, with a very long "tail" on concentrations in nature (with or without bumps). As Campbell and Deffeyes discuss at length in their books, unlike metals, petroleum is destroyed both at depth and at the surface ("petroleum window"), and there was only one way to produce and trap it, during very limited periods of Earth history, under very, very special conditions. When it's gone, it's gone. Coal likewise. In contrast, metals like copper, for example, are never destroyed, only redistributed, and there are many, many different geological ways to concentrate and preserve them as ores in nature. As you and others have noted, in the event of a collapse, the metal miners of the future may be digging mainly in old landfills.
Yes, Deffeys is doubtful that Skinner's barrier exists as a general feature. It is also true that we lack complete data of concentration vs abundance for most minerals. But I think that Skinner makes a very convincing case in his papers. Uranium is something special, for one thing it doesn't form natural sulphides; the most common case of ores. Anyway, as you say, the mineralogical barrier is a limitation to minerals availability, but the problems would remain even if it didn't exits. Thanks for the note about diamonds from the seafloor!
Thanks for your response. Although not a sulfide-former, uranium does form extremely concentrated oxide ores, some so concentrated that they have even gone critical in the past (Oklo phenomenon), so I'm not sure how atypical it is as an ore-former. Many other useful rare metals (e.g., tungsten, tin, beryllium, vanadium, chromium, thorium, lanthanides) likewise don't typically form sulfide ores, and likewise vary greatly in their concentrations in rocks.
My main concern is that you may be making the mistake of the economists in reverse - they assume that petroleum must have a very long concentration tail like metals do, and therefore can't peak if the price goes up sufficiently, whereas you seem to be assuming that all metals must behave like petroleum, with little or no such tail at ore grades, and therefore must peak. Peak energy availablility alone seems reason enough for peak metals, without assuming a poorly-documented bimodal geological underpinning (which might well be valid for some metals, such as mercury, the only liquid metal, but not for others).
Re the comments on mining the sea floor & black smokers. As well as the undersea Namibiam off-shore diamond mining, the mining of undersea copper sulphide deposits from these black smokers is not as far off as you may think. See www.nautilusminerals.com website. It is less than 5 years away.
All we need is the universal minning machine and that enzime that breaks the molecular structure of Cellulose fiber allowing production of human digestable foods and we are good to go.
Fungi. Oyster Mushrooms grow on a wide base of cellulose.
Example - Oyster Mushrooms on straw means you can take the fungi-covered straw and feed the straw to cattle. (Yup, conversion of a 'not edible' to 'edible')
Great post Ugo,
Your post got me wondering at what concentration of uranium would the energy from the uranium no longer repay the energy used to extract and process it. Obviously in concentrations it is found in seawater the energy used to extract it would far outweigh the energy from the extracted uranium but since it is an extremely energy dense element i was wondering if anyone could give me a simplistic ballpark figure to satisfy my curiosity. (1%, 0.1%, 000000000.1%etc).
Pulverizing hard rock has an energy cost near 50 kWh per tonne if it is to fit 80 percent through 25-micron holes; half as much if the holes are 100-micron. Uranium yields 54 kWh of electricity per gram in the CANDU reactors nearest me, so if only 1.08 grams per tonne can be extracted, it might all go to power the crusher. Average rock is 2.2 to 2.8 grams per tonne, so it probably can yield a little net energy in this case. There is about as much CANDU-accessible fission energy in the average underfoot rock as there is tar combustion energy in the Alberta tarsands, and that energy is two-thirds net or more.
Seawater extraction of uranium definitely yields a very high net energy fraction, because nothing laborious need be done to the water; it could be considered pre-crushed. The uranium price at which this was expected to pay in 2001 was briefly surpassed recently, but now prices have settled back down. The rate of discovery of very cheap uranium in Australia in 2007 was nearly equal, in thermal terms, to the whole world's rate of petroleum burning.
How shall the car gain nuclear cachet
Physicist Michael Dittmar showed this in his ASPO VII presentation (slide 12):
With desalination becoming more common in some parts of the world I have wondered wether mineral extract from sea water could piggy back on this?
As the minerals are concentrated in the brine from desal plants would it not be more viable to attempt extraction from this water?
I think you'd have to consider details of the desalination method. My impression is that most desalination plants don't produce a very concentrated brine. You might have more luck recovering metals from evaporation ponds, such as those used for sea salt production for thousands of years.
Lets ignore seawater for a little bit; The energy cost of the rossing mine in nambia is 1/500th the energy returned in light water reactors from the uranium mined, and the ore grade is 300ppm.
Then consider that breeder reactor regimes are 200 times as fuel efficient than light water reactors.
The energy from mining uranium will never be a showstopper in nuclear power production.
See disturbing photos of Mountaintop Removal coal mines at:
http://www.ohvec.org/galleries/mountaintop_removal/007/index.html
Thanks for this link, I guess.
As some one who frequently camped in So. WV, it depresses the hell out of me to see this.
Know what you mean, I think.
According to Fred Mooney, an active member of Earth First!,
There are more shame-inspiring, logic-defying, melancholic stats, photos , diagrams at:
http://www.mountainjusticesummer.org/facts/steps.php
They mine coal there, right?
With the coal you produce electricity, right?
So, when you bemoan the loss of the beautiful nature there, can you digest these facts (source):
- One demand at google takes as much electricity as 1 hour of an 11 Watt light bulb.
- One figure in "second life" needs (in average) 1750 kWh, which is more than needs an (average) indian citizen per year.
- The youtube servers today have as much band width, as the whole internet in 2001.
- The internet produces as much CO2 as the entire aircraft industry.
I think we will all see us in hell... :-(
SNOMM
To get an idea of the size have a look at this and see if you can see where the bulldozer was parked.
http://www.techeblog.com/index.php/tech-gadget/a-look-back-worlds-larges...
Some sobering thoughts for when today's mining boom ends and we are left with holes in the ground where rich deposits used to be and energy is too expensive to pursue lower grade deposits. In a crisis or military escalation energy costs could get subsidised for the 'common good' eg nepheline for aluminium in Russia or greensands for potash in the US.
A cornucopian view I've heard is to 'dig deeper' since there is no reason to suppose the best deposits are close to the surface. I also wonder whether mining viability is a dynamic variable not static. Specifically China's 10+% economic growth means the money will be found for strategic metals hoping that unit costs will reduce. A few weeks ago I saw a nickel mine and a zinc mine only walking distance apart (google Avebury and Comstock) competing for labour and materials to build processing plants. If China nosedives perhaps both will close.
Finally the carbon/metals complementation. I predict that despite Australia signing Kyoto the powers will say 'we have to dig up more coking coal otherwise we can't sell iron ore'.
Well I can think of one cornucopian source. If the price becomes high enough, we could buy products that contain the mineral/metal and recycle it. Of course when the product is identical to the end product you got yourself a problem. But in this manner the resource will migrate towards the most valuable uses.
I recall reading something more than 50 years ago, that actually seems non-intuitive and may be totally wrong based upon what may be a faulty recollection. But, the point was that with all of the gold mining that has been carried on for the last thousands of years, the total amount of gold from the earth could fit in a cube that is 50 feet on each side. Does anyone know whether this is right, or wrong?
You're probably right. In my old lecture notes on gold, I have as of 1988 it would have been a cube 55 feet on a side, which corresponded to a total world production of 3.1 billion ounces. I'm not sure of the original source of this statistic.
Not a bad estimate for 50 years ago, give and take a few inches!
Today the cube would measure about 67 feet each side (including all the tooth fillings, jewelery in India, industrially used and the stolen gold in the WTC bullion heist.)
[Total of about 164,389 tons ever produced -- overground gold end of 2007 -- based on 2001 World Gold Council estimate and other data since.]
One aspect of mining and energy that I have yet to see discussed much here (in the relatively short time I've been reading TOD) is non-metallic resources - the very mundane sand, gravel, and limestone required for concrete production, the clays for glossy paper, and so on, gradually increasing in value per ton up to, say, diamonds. These commodities already are comparable in value and total tonnage moved to mining metals, and their total cost, in general, already largely reflects energy costs, especially for commodities mined in bulk (why most gravel and crushed rock quarries supply only local needs). At what point then does it simply become too expensive, in terms of materials costs, to build more cities and highways (even with cheap labor)? Will "peak concrete" necessarily coincide with peak oil (or peak gas, or peak coal)?
Of course not. Limestone is everywhere, and energy for refinining it is avaliable from the sun (10^16 watts) and from the ground (160 trillion tons of fissionables)
True, but consider how incredibly cheap construction materials have to be, owing to the tremendous volumes used. Already, even with cheap oil, the main aggregate cost is haulage (you just dig it and haul it, perhaps with some intermediate crushing and screening, unlike the very fancy processing required for metal mining). For the cement that holds the aggregate together, add in the roasting (and environmental) cost of driving off the carbon dioxide in limestone. Recently, where I work, I heard that a new building had abruptly increased in estimated cost by nearly 50%, putting it well over its budget. This increase was described as mainly caused by the increased cost of concrete, presumably a direct reflection of increased energy costs (inasmuch as the construction industry itself is slumping).
Your solar energy or fissionables might have to become as cheap as today's fossil fuel burning in order for construction at today's scale to remain affordable to public and private entities. Otherwise, peak concrete might be implied, regardless of angst in suburbia, decreased driving, and similar PO effects. Of course, for all I know, environmental regulations may have us increasingly building with, say, glass and glass-cemented aggregate, instead of concrete, long before the cement itself gets too expensive. That's because Portland cement made by decarbonating limestone is a relatively recent invention, and concrete, although currently cheap and practical, is neither the most environmentally friendly nor the longest-lasting building material out there. (It may also be one of the ugliest, but that's subjective.)
There's no evidence of that. A century ago, energy was far more expensive in man hours and the ratio of the economy than it is today and civilization still boomed.
Er, you've completely lost me. No evidence of what? At the moment, we're not debating the future of civilization, but rather the future of mining and materials usage. A century ago raw materials usage ("concrete" in the present debate), even on a per capita basis, was far far less that it is today. That is because energy then was far more expensive, as you correctly noted. If energy again becomes relatively more expensive, this materials usage may have to change downwards (become more energy efficient). In other words, peak concrete. If new forms of energy (your solar and fissionables) can completely take the place of FF, in a timely manner and at comparable costs, then perhaps we can afford to continue building endless giant, widely scattered, energy inefficient concrete homes, buildings, and highways. In other words, there will be no peak in the usage of concrete (or ceramic substitutes). Are you so confident of that wasteful energy outcome? Remember, peak concrete need not mean the end of using concrete (to paraphrase a mantra heard often on TOD). Did you perhaps misunderstood me in that regard?
I started by postulating that the price of concrete and other bulk raw materials should closely reflect the price of transport energy (FF in today's economy), because they have no intrinsic value while still in the Earth, and precious little value while still at the quarry or mine site. The highly profitable construction and housing industry depends on transporting, inexpensively treating, and shaping these formerly valueless commodities into various sizes of boxes, adding a roof plus minor wiring and plumbing, and selling the expensive result to rich people, companies, or governments. The economies of whole cities and states depend largely on this growth industry, which currently is shrinking dramatically (at least, outside of Asia). What is it that you disagree with? Again, sorry, but I'm lost.
Are you so confident of that wasteful energy outcome?
Yes.
Very nice post. My reaction to it tonight is mostly philosophical.
Humans are the closest thing to a universal mining machine this solar system will see, and we're about to wind down.
Inasmuch as we as a species indulge in negligible future planning (high discount rate), we have with nearly no exceptions resorted to "best first" when exploiting energy and minerals, which is to say "easiest/most profitable" first. Thus, the only thing which has kept us going has been fresh horizons of information about where resources are and advancing methodologies.
It's my seat-of-the-pants impression that we're well into diminishing returns on all aspects of this now: we know to a reasonable approximation where the 'best' stuff is, we've made most of the reasonable tech breakthroughs, the EROEI of our energy sources is declining, we are working ever-poorer ores, and the globe-spanning extractive empire is now holding itself up by its own bootstraps as that empire itself suffers Tainteresque diminishing return on investment in complexity. My guess is that much of the current mining infrastructure would have difficulty restarting if it were ever stopped; it is efficient, not resilient. And pretty much all the parts have to work to leverage all the other parts at this point.
The global extractive infrastructure we have evolved is not universal, but it is the ultimate mining machine for this planet. It will run awhile yet, and progressively not in a nice way as we funk towards low EROEI and crappy ores in a lower-complexity infrastructure with increasingly large and poor populations of humans working the pits. The ready availability of rare minerals in pure form is a luxury our descendents will have to do without... with all that implies; unless the ultimate mining machine precludes their very existence as a side-effect.
It's not a bad approximation to say that humans evolved to find concentrated stuff and un-concentrate it. Nascar, salad shooters, culture, war, sex, religion... that's what they come down to. Brain stimulation for us, catalysis for planet earth.
Y'know, we really should shut the thing down and spend a thousand years reassessing our options. A mine is a terrible thing to waste.
There is nothing worse than a closed mine.
How shall the car gain nuclear cachet?
Nice pun, although an ambiguous one. If you want to be geological as well as philosophical, a thousand years of uncontrolled erosion and sedimentation would probably alter most of our mistakes beyond recognition, except perhaps in deserts, and whatever ones were left would be completely ironed out by plate tectonics in a few hundred million. New mines and new oil would be created for whatever form of life could then take advantage of them.
Actually it was a weak pun, but that's how it struck me, a "my poor Krell" moment about what yeastlike dumbasses we humans seem to be. I seem to recall that M.K. Hubbert noted that if the USA were smart it would save its own oil for last rather than exporting it, and in that same vein the "best first" approach to resource extraction is greatly sub-optimal in terms of sustaining human populations and well-being, it's really an aspect of the tragedy of the commons.
Your point about nature covering up our mistakes seems to miss my point, which is probably my fault. I wasn't speaking about the aesthetics of holes in the ground but of the fate of our species. And other species. And I don't wear my geologist hat much these days, but plate tectonics isn't going to alter minable mineralization all that much in the next million years. A few hundred million maybe, but the odds on the rise of another technological species are near-nil and that may be a good thing.
No, my lament was simply the sniveling of a self-aware cancer cell wishing vainly for a remission by its cohorts. Despite Jay Hanson's fine work, this was perhaps not all inevitable, and is perhaps not inevitable now. I recall the perhaps-apocryphal story of how American Indian children of some tribes were taught never to hunt porcupine, because no other predators could eat them and any human could kill one easily with a stick at any time, so they would be plentiful during famine. This is the opposite of "best first" and amounted to leaving intentional resilience in the system, and it's only one example. If you contrast that with, say, Europeans arriving on Mauritius and finding Dodo birds, they beat them all to death with clubs despite not wanting to eat them, just because bashing them with clubs seemed a cool thing to do. The tragedy of the commons has a strong cultural element. (universal "mine"?)
It'd be nice if we had helium and indium and other stuff in a hundred years and a thousand; there might be actual uses for it. It'd be nice if we didn't burn enough carbon to phase-shift the earth into anoxic-ocean phase. It'd be nice if we considered our descendents and stopped doing the yeast thing. For there are a LOT of things worse than a closed mine.
Thanks for your explanation of the complex pun (basically my "mine" is what's mine, if I understood you). I agree with your final statement. Personally, I remain confident that no matter what we do to ourselves and the world around us, life will find a way (quoting Jeff Goldblum in "Jurassic Park"). The next top predator may be a giant wasp, or an intelligent cockroach, or a giant rat, but there will probably be one. In this regard, societal insects have been far more successful and stable in the long run (hundreds of millions of years) than societies of humans ever will be, especially those societies that deliberately encourage selfish, immature thinking (I want all of what's mine from my mine right now, to continue the play on words).
I wasn't going for a complex pun, but in english it does seem to work that way.
The evolution of a new 'top predator' will certainly occur unless we manage to turn the earth into venus, but that's a far cry from self-awareness, and I'll bet a nickel that they won't be mining molybdenum. Life will do its best, but this may be the one shot the neocortex gets. I've done a lot of work with dolphins and other species and it's a big shame that we may end their evolutionary lines as well. Theirs is the real tragedy, ours being self-inflicted.
The insects will survive us, but that's cold comfort. Now if we could emulate a few of their stragies in lieu of mining out the world, we might have something. Synthetic spider silk comes to mind as an advanced structural material which wouldn't require a lot of mining. They have spider-goat chimeras now which create silk precursors in their milk. Whether that's a step away from mining, or towards the next formidable top predator, we'll have to see.
You need to bear in mind that the hydrothermal and volcanic processes that form mineral deposits are on-going, thus in a few million years time we will have a new inventory to plunder. More important are the processes of weathering and erosion. 2 cm / year in some places - that can remove 200 m overburden in 10,000 years - exposing new deposits and making current deep uneconomic deposits appear at surface - philosophically speaking of course.
Much of the metals we mine are not actually consumed and are available for recycling - Manhattan may become the new Eldorado. With falling population, we may find we have an an embarrassment of riches left by our forefathers - that can be recycled at much lower energy input than the initial mining - using solar energy of cousre.
Agree completely, as indicated by some of my earlier comments. Moreover, in deep underwater submersibles, we can observe the formation of metalliferous sulfide deposits at mid-ocean ridges in real time.
Sometimes (oftentimes?) we can get something from an ore which is too low grade, because it is a byproduct of something else. So if you are interested in say silver, you may still get some production from ores that are too low in concentration to profitably mine silver by itself, butthat you may be mining for say lead. I suspect the amounts aren't that large however. So at some point we begin digging up our garbage dumps, and mining them for discarded stuff.
Actually, for the past 40 years or so, lead has been a limited-value by-product of silver mining. Lead batteries are required to be recycled and lead-based paint is illegal. Only the huge run-up in Asian new car ownership has rendered lead somewhat in demand. Your general principle about synergy (co-products and by-products) in metal mining is correct, though, and the amounts produced can be large. During economic downturns particularly, the low-cost producer rules (keeps on mining), and by-products help a lot.
What is lead used for these days? Lead acid batteries seem to be on the way out. It is no longer allowed to be added to paint or petrol. It was used a lot for waterproofing roofs, but I don't know if it still is. Perhaps containing kriptonite safely?
Well, bullets are still legal, although some governments have outlawed lead shot for shotguns, replacing it with less effective steel shot. Ammunition represents only 3% of usage, however, whereas batteries represent 88%, with the rest fairly miscellaneous (radiation shielding, solders, caulking, glasses, ceramics, and so on). This is my reference:
http://minerals.usgs.gov/minerals/pubs/commodity/lead/
You could devote a book to Mitigation strategy 4: Re-use
It is the most unaccepted topic in a capitalist run world. It is also the only logical answer.
It doesnt mean you have to make things ultra long life. It means things have to be STANDARDISED, SERVICEABLE, MODULAR. So you can go to the junk box in your shed, pull out the cable you saved when you disassembled the washing machine and reuse it in your car. It means 6 wheel nut layouts from skateboards to monster trucks, 20 standard tyres, 1 standard phone charger......In reality very hitech things would get slightly bigger and clunkier - big deal!
Only governments or international groups can make this happen. It needs to be a voting issue. One less effective but simpler way is to tax new goods so that servicing costs become lower than replacement costs. I am aware, of course, that we will fry before that ever happens. It also just makes a gravy train spare parts industry.
Consider this - this thread is only here because of HTML, ethernet, SCSI, IDE etc. [and the older standards of 240VAC50Hz and 120VAC60Hz etc]. Remember before those?
Remember before those?
The ASCII and RS-232 standards.
Go to the top of the class.
In those days the chance of your Joe90 mainframe talking to my roomful of ICL mainframe without both manufacturers secret cables, dongles, widgets, gateways and handshakes was zilch..
In relation to the German Garzweiler coal mining giant, NASA climatologist James Hansen has written a letter to Chancellor Merkel
http://www.columbia.edu/~jeh1/mailings/20080122_DearChancellor.pdf
and writes:
Outstanding article.
Thank you very much, I enjoyed reading it.
The Machinery's Handbook has been the machinists' bible for about a hundred years. It has industry and government standards for everything from steel composition to light bulb thread sizes. Too bad we don't follow the standards we already have. Didn't somebody come up with the idea of interchangeable parts a while back? Didn't catch on, I guess.
Jon.
We don't need Universal Mining for asteroid mining. The same metorites we used for iron are out there in massive quantities, just waiting for us.
Just a matter of someone throwing money at the problem. The non-renewable energy cost isn't even that high. If mining asteroids is more cost-effective than mining landfills (and it probably is), it will be the solution.
The one thing I never see here is mention of orbital and other space-based solutions to our problems. Orbital powers stations, whether you send it to earth as microwaves or plain old extra sunlight is irrelevant. Beanstalks for cheap ground to orbit shipping. Asteriod mining. Hosts of other things.
All true, but the energy-investment thresholds for this are so high and the payoff so deferred that human civilization simply won't do it. It's thermodynamically possible but not evolutionarily possible at this point. And that's a great shame.
What, no mention of Von Neumann machines?
Here's a nifty page with pics of the Krupp "Bagger 288" excavator - the world's largest machine (short of the CERN supercollider). Dark Roasted Blend: The Biggest (and Hungriest) Machines. Crazy big - puts the NASA vehicle trawler in the shade!
The figures for recycling are old (2002). With commodities metal prices rising 100% to 300% over the last five years much higher percentage of metal is recycled now. My friend in the metal recycling business says:
Steel 60% + recycled
Copper 70% + recycled
Don't know for aluminum or stainless steel but these have to be higher since scrap price for both is up 50% over a couple years ago.
The assumption of cars lasting longer if made of stainless steel is largely correct. But I believe average life of a car would still be under 25 years due to technology changes in various systems on the car and rebuilding propulsion/suspension would eventually outprice buying a new car.
Corrosion free car bodies would extend their life indefinatly. In Southern Africa with its dry climate you saw imported cars 30 years old still chugging around. In damp climates like Britain they become rust buckets in a few years. With non corrosive bodies the only reason to scrap a car would be to get advances in technology.
We could run solar and wind power uranium/seawater separation plants to get uranium to run nuclear reactors. That's how we could get power for computers during the night? Or would it make more sense to heat underground aquifers and make artificial geothermal power systems?
Just a philosophical answer to a philosophical question about what we are going to do when the low grade ores run out in a few hundred years.
Ugo, excellent, really excellent post. A complex thematic laid out for the common man.
Sir Fred Hoyle once said:
I still believe that we can accommodate ourselves somewhere in between mining the Asteroid Belt and Subsistence Agriculture, in a setting more interesting that today's exponential growth society. But I'm young, I'll reach retirement age after all the Conventional Fossil Fuels peak; perhaps I can't afford thinking any other way.
Ugo - nice article - keep em coming. A few random comments:
The Plasma Quad Machine
In all mining operations its important to know which minerals the elements of interest reside in. When you cross the mineralogical barrier the elements will tend to be dispersed in microscopic accessory mineral grains - that are impossible to separate - or as trace elements in silicate minerals. The only way to tackle this is by dissolution (HF) or fusion of the whole rock. One advantage is that you would get a chance to access the periodic table - the dissadvanatge is that you would need a mass spec to separate the elements / ions. You may find problems maintaining vacuum doing this at an industrial scale.
Uranium
Uranium mineral deposits form as a result of the two oxidation states of U ions. In its oxidised state (6+) U is highly soluble in water. In its reduced state (4+) it is completely insoluble. Thus U deposits often form at a redox boundary in the crust. I'm a little surprised to hear that a bimodal distribution does not exist for U ores and would treat that information with some scepticism.
The other crucial thing with U ores is the presenece or absence of ore minerals - uraninite et al - that are soluble in weak acid - thats how you get at the U. In many lower grade rocks the U will occur in zircon (silicate) and monazite (phosphate) both of which are rather indistructable.
The Scum of the Earth
The Earth's crust - where all our mineral deposits occurr - can be viewed as scum floating on top of the mantle. All the incompatible elements that we want to mine don't fit into the latices of the olivine and pyroxene that make the mantle - which is depleted in scum.
Asteroids (meteroites) tend to be formed from the depleted interior of the former planet that lay between Mars and Jupiter - so you can well and truly forget about mining this - apart from the iron meteorites - which are the remnants of the metallic liquid core.
Time scale
The average age of thermogenic oil and gas on Earth is likely to be of the order 100 to 200 million years. Biogenic methane is much much younger.
I'm not sure about the average age of mineral deposits - everything from brand new for those forming on the ocean floor today by chemical and hydrothermal processes to very ancient - many of the platinum group deposits are Proterozoic and Archaean in age - billions of years old.
Die-off
On a planet with declining population there may be an embarrasment of riches left on the surface by prior generations. The Chinese I believe are taking recycling to a new level employing hoards of cheap labour to dismantle consumer products.
Euan, a few comments to the comments
Plasma Quad (or TOF). Yes, it would be very difficult; it is a conceptual experiment. If it is ever done, I think it will be done by mechanical and chemical means - very difficult anywqy
Uranium. Indeed, it is surprising that it has a non bimodal distribution. The explanations given are, in my opinion, not very satisfactory. The only one that makes sense to me is the lack of stable sulphides of uranium. But, still, uranium oxides do tend to form deposits. Anyway, the only source of data on this point is Deffeyes's book. The data reported seem to make sense, but haven't been verified, validate, or reproduced
Scum of the earth. Indeed. The scum floats and it is from the upper crust that we get minerals. Some economists have written very funny papers assuming that you can mine at depth of tens or even hundreds of km. No way.
Time scale. The time scale for forming mineral deposits is on the average in the range of the hundred of millions of years. However, for most of the time, nothing happens. When the process starts, a mineral deposit or an oil or gas field can be formed in a few hundreds of thousands of years
Die off. Indeed, our descendants will have great abundance of metals in reduced form. For them "mining" will mean mining the remains of our civilization. Think of the Amish; I bet it will be something like that. Or, else, those pesky belters with their torchships (now, which sci fi author wrote that? Can't remember.)
And comments on those:
Uranium is different enough from most of the elements in granite that any of it in excess of what can be accomodated in minor amounts in trace zirconium and thorium minerals tends to form its own oxide mineral, uraninite. The reducing conditions needed to stabilize insoluble uraninite are provided by the magma itself - you don't need the organic matter that tends to precipitate uranium from groundwater in sediments. Ordinary granites can vary greatly in their radioactivity, and the higher readings are generally provided by excess uraninite - an easily separable ore mineral. I believe that this type of occurrence is the basis for Ken Deffeyes claiming that uranium ores have a regularly declining tail, rather than the deep intermediate valley proposed by Skinner.
Many other valuable metals behave like uranium. They don't fit in the crystal structures of common minerals in granite (quartz, feldspars, and micas, mainly), and so the process of fractional crystallization of granite as it cools (similar conceptually to progressively crystallizing various salts by evaporating a brine) concentrates these elements in the residual silicate melt (magma). The geochemist calls these elements "incompatible" because they don't fit. Such elements include lithium, beryllium, tin, tungsten, molybdenum, fluorine, and several other less common ones. Most of these, like uranium, don't typically form sulfide ores, although molybdenum does. Prospectors know that they can find these minerals associated with silica-rich, light-colored, radioactive igneous rocks (granite and its relatives).
Another groups of metals, such as manganese, nickel, vanadium, and chromium, tend to substitute for magnesium or iron (in its different oxidation states) in silicates such as olivine or in oxides such as magnetite or chromite. Their concentration likewise varies greatly, and they likewise need not occur in sulfides (although the highest grade nickel ores, commonly with associated copper and platinum group elements, commonly are sulfides). Prospectors know to find these elements associated with dark-colored, silica-poor igneous rocks (gabbro and its relatives). I suspect that the concentration of these likewise would not display Skinner's "double hump" pattern, although I can't demonstrate this. Nickel might be an exception, in part.
The above two examples treat ores formed by igneous (magmatic) processes. Without rereading his papers, I believe Skinner was thinking mainly of sulfide ores that tend to be deposited in veins by hydrothermal processes, such as copper, zinc, and lead. If you are in the vein, you have high grade ore; once outside of it, you don't (except for trace amounts). Very simple. Of course, precious metal ores even in this category most commonly are mined at the ppm level. 1 ppm gold (1 gram per ton) can be economic for gold in certain types of deposits, for example.
I suspect that generalizing about metals could be as dangerous as generalizing about individuals in various large groups of people. Chemistry in the case of metals, and genetics in the case of people, tells us that all are different.
Scum of the Earth: We mine the surface of the Earth's crust, and the shallow subsurface, because that is what is accessible to us as surface dwellers. That doesn't mean that certain types of ore deposits can't and don't form very deep (diamonds for example). The first or "incompatible" group of metals, including uranium, tend to be concentrated in the Earth's crust, the second group is just as likely, if not more likely, to be found very deep in the mantle. We can't mine them there, but that's where most of them probably are. Hydrothermal processes depositing sulfide ores in open veins can only affect the shallow crust, of course.
That reminds me of issues people were having in
Iceland with geothermal heat extractrion.
The heated water would come up from the depths and
as it cooled it would dump out dissolved minerals
all over the pretty heat exchanger.
Interesting.
It was Robert Heinlein.
Do your energy estimates include that if we mined average crust rocks, we could extract all the minerals at the same time?
Of course, the distribution of the rarer elements would be less than ideal, and again I wonder how that compares with industrial usage?
The figures given for recycling also seem to be based on US data - I suspect that re-cycling rates in Europe with the latest legislation are far higher.
If you mined the asteroid belt you would also hardly transport everything back to earth, but rather any rare elements that were scarce in the crust.
So essentially my question would be with low rates of requirements of mater4ials due to state of the art recycling, the mining of all basic metals simultaneously from the crust, and the very low volume transport of rare elements back to the earth, could we maintain the present civilisation on a reasonable energy budget?
And my comments on your excellent one:
Plasma quad machine: Agree you have to know the mineralogy of the ores. Maintaining a vacuum would be trivial in space, as mentioned in an earlier post.
Uranium: See my comments on Deffeyes vs. Skinner. Related to phosphates, the most common phosphate mineral, mined apatite (also what our teeth and bones are made of) likewise accomodates minor uranium in its crystal structure. This uranium is commonly separated in making phosphate fertilizer, along with fluorine used for treating drinking water (so that it can go into the apatite in our teeth, reducing tooth decay).
Asteroids: Not my field, but I understand that there most likely never was a planet between Mars and Jupiter. Most meteorites that strike the Earth, and presumably most asteroids, are said to be "primitive" meaning they never were part of a broken up planet. Some probably originated in the outer solar system. Others, including the rare metallic ones, do appear to come from a somewhat differentiated planetary object, but their parent probably was no larger than some of the larger present-day members of the asteroid belt.
Time Scale: Although some mineral deposits can be billions of years old (especially in the most ancient areas of continental crust in Africa, Australia, and Canada), most are much younger, simply because the older ones have all been eroded or subducted (and because typical hydrothermal ores in veins only form in the shallow crust). Renewal of many types of mineral resources shouldn't take long geologically, but the times involved are longer than human lifetimes, and so need not concern us much.
This stuff about uranium in seawater seems to be going off the deep end. It might be easier to get it from coal.Does anybody have any data about the concentration of uranium in coal? Would it be better to mine coal its uranium content rather than burn it? If the Uranium content is high enough coal with an EROEI too low to be mined for other uses might be viable for it's uranium.
But the experiments were done, on Japanese government money. That government takes very large revenues on petroleum, and I guess on imported LNG also, so those whom it employs, and assigns to do research into inexpensive domestic energy sources, presumably understand that they hadn't better find any.
Maybe seawater uranium seemed like a pleasantly unpromising avenue for that research; the concentration is, after all, barely over 0.003 ppm, and water-cooled reactors get only ~600 GJ/kgU, so with all the U in seawater they can heat that same seawater by about 0.4 K, or with electric pumps lift it about 60 m.
However, adsorption of dilute materials by slow flow of very low-viscosity fluids over large adsorbing surfaces has tremendous extractive power. It's how green plants get atmospheric [CO2] at ocean monitoring stations down almost as low, at the end of each northern-hemisphere growing season, as the same time the year before.
Water has about ten times less kinematic viscosity than air. Cargo ships that float in air have greater drag than those that float on water, and AFAIK have never existed; only passenger liners, the Zeppelins, ever floated thus. Also, an adsorbed UO2(2+) ion is an energy haul 360,000 times larger than the solar energy that a plant's capture of a CO2 molecule enables it to store.
Maybe if someone had pointed these two facts out to the funding bureaucrats, they wouldn't have funded; it might have seemed to them like looking for uranium on land. But they did. It wasn't their fault that reality itself decided to go "off the deep end", and marine uranium extraction turned out to require so little energy that it would yield net energy even for parts per trillion of U.
How shall the car gain nuclear cachet?
Ugo Bardi, one of the best posts I have ever seen on the Oildrum. Very well done. It is like a synopsis of "Energy and Resource Quality" by Hall, Cleveland and Kauffman.
Got a far as the peak lead chart...
Lead is highly recycled...let's say 100%. Then only new incremental use is required of new production.
Looks less like a peak than more recycling and perhaps a slow down in new demand.
you need to do more research on melting points for many metals and their man made blends. for example compare the common steel washer to a building grade steel I beam. the former will easially be smelted, the latter can only be softened inside the best non-industrial blast furnace(which was the Japanese one used to create the low quality* steel for katana's).
Aluminum though will be completely unworkable since it's melting point is over 2000F. to put it bluntly we are going to go back to iron and low quality steel(in non-industrial amounts)
*low quality because the steel produced is filled with impurity's that render allot the metal useless. it also has a un-even distribution of carbon which means after the creation of the steel the master forger sorts the pieces so he can have both the low and high carbon pieces of steel to form the katana. because of this the master smith would reject the whole batch of steel. It's because of modern movies and shows that the common myth that japan was filled with samurai's wielding katana's same with Europe and armored knights. but in reality due to the great difficulty getting good steel katana's were limited to only the rich and powerful.