Natural gas, the green choice?
Posted by Chris Vernon on June 24, 2010 - 10:26am in The Oil Drum: Europe
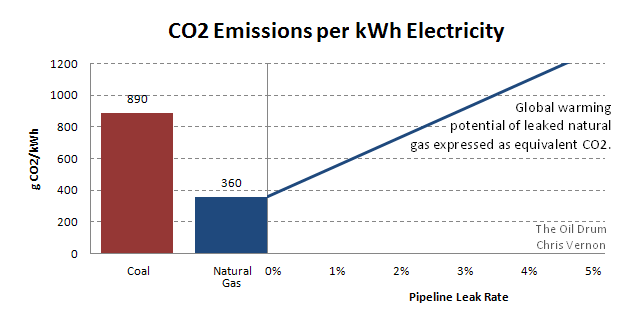
On the left, CO2 emissions per kWh for coal and natural gas. On the right, the global warming potential of leaked CH4 expressed as CO2. With a leak rate of 3%, gas fired electricity has a similar global warming potential as coal.
The figures below are for the UK electricity grid.
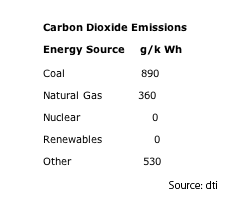
This table was lifted from: http://electricityinfo.org/co2emissions.php
These CO2 emissions are directly related to the fossil fuel combustion and power station efficiency. Lifecycle emissions are not included, leaving nuclear and renewables at zero, because emissions related to construction, decommissioning, uranium processing etc. are ignored. Natural gas is considered the ‘greener’ fuel as electricity from gas emits 2.5 times less CO2 than coal, as well dramatically lower CO, NOx and virtually no SOx or particulates.
There is an issue of system boundaries here. The figures above only consider the power station and not any upstream supply system. While CH4 may leak from the gas pipelines, there are also CH4 releases from coal mines. For this post, let’s consider emissions after the mine mouth or well head, and ignore emissions associated with transporting coal.
For oil and coal, the only significant route into the atmosphere is via combustion. However, besides being burnt, natural gas can be released without combustion as methane, CH4. This becomes interesting when one considers both the impact of atmospheric emissions of CO2 and CH4. Both are greenhouse gases in that they that absorb and emit radiation within the thermal infrared range of the electromagnetic spectrum, however their respective radiative forcings are very different. The radiative forcing measures how much a greenhouse gas (or other factors) alters the balance of incoming and outgoing energy in the Earth-atmosphere system.
The Carbon Dioxide Information Analysis Center (CDIAC) part of the US Dept. of Energy uses Global Warming Potential (GWP), as it provides a simple measure of the radiative effects of emissions of various greenhouse gases, integrated over a specified time horizon and relative to an equal mass of CO2 emissions. Over a common 100 year time horizon CDIAC state the global warming potential of CH4 as 25 times greater than CO2 [link]. The calculation is not trivial, and estimations do vary a little, but for this analysis the factor 25 is sufficient.
We saw above that natural gas emits 2.5 times less CO2 than coal when used to generate electricity. However, when CH4 is released to the atmosphere without first being combusted, the global warming potential is 25 times higher than CO2. It is a more potent greenhouse gas. If only a little natural gas is released without being burnt, it will dominate the radiative forcing and more than compensate for the 2.5-fold advantage gas has over coal.
The chart illustrates this effect:

On the left, CO2 emissions per kWh for coal and natural gas. On the right, the global warming potential of leaked CH4 expressed as CO2
If the natural gas leak rate is 3%, the global warming potential of a kilowatt-hour of electricity from gas is equivalent to coal.
Leak Rates
So what are pipeline leak rates? A 1997 US Environmental Protection Agency report states US methane leak rates were 1.4 +/- 0.5 % in 1992. The largest source of leakage at that time was compressor components used in the processing, transmission, and storage, followed by the distribution network itself, with the small length of old cast iron pipes leaking disproportionately highly. The natural gas production process also contributes through millions of slowly leaking pneumatic control devices. A larger study carried out from 2005 by Brazil’s largest gas distributer Comgas suggests cast iron pipe leak rates double the EPA study.
A 1990 study for Greenpeace considered the UK distribution network then operated by British Gas. Greenpeace estimated low, medium and high scenario leakage rates of 1.9%, 5.3% and 10.8% respectively. This was in contrast to the 1% claimed by British Gas at the time. The authors were confident leakage rates were above 1.9%. These figures are likely obsolete today as there still existed a large amount of pre-1970 cast iron pipe work, much of it since replaced. In 1990 only 39% of the UK mains and 74% of the service pipes were plastic.
The 1.4% figure is also old, and only refers to the US, but it is a significant magnitude, it represents a 70% increase in global warming potential compared to the CO2 alone and halves the CO2 advantage gas has over coal based on the 360 and 890 g/kWh figures above.
Whilst these figures do not tip gas beyond coal, they halve its advantage. They are also only national. For the US this is quite understandable, but for the UK and Europe, the gas system is changing. Could leak rates become important as natural gas supply routes become longer? As Europe increases its reliance on Russia, as previously stranded gas is brought to market through longer pipelines than before, as a larger number of smaller deposits are exploited and as existing infrastructure ages, it seems likely that leak rates will increase. We often hear about struggles in the former Soviet states related to gas – is the leak rate there one percent or five? Is it economically feasible for the pipeline operator to make investments to stem the last percentage point of a system's leaks?
Is it possible that a ‘green’ gas power station in the UK is making a greater contribution to global warming than one burning coal?
Does anyone have recent data on leakage rates, especially for Russia and Eastern Europe?



Very informative, but mining coal also releases methane. Assuming that most methane coal bed gas just gets ventilated out, what does that do to the comparative GHG contribution of coal?
Excellent point. We measured the concentration of Methane out the vent in a Southern Illinois Mine and converted to a daily rate of 4 MMscfpd. They spray the walls to keep it down.
FF
FF, how does spraying water on methane keep it down? Coal dust, maybe, but not methane.
I've not been a fan of mine-mouth powerplants until now, but if such a plant was necessary to process the exhaust air from the mine face to burn the evolved methane... that's mind-blowing.
For sure, any idea how much that is typically, per tonne of coal mined? I don't know which is why I closed the system at the mine mouth/well head (and ignored greenhouse gas emissions associated with coal transportation, assuming them small).
Yes, but this would tend to skew the equation in favor of coal. Ideally we could get estimates at every level of production, transportation and use (how often do people leave their natural gas stove top range on unlit...)
I suspect that ultimately this could put us in the same predicament as with our attempts at fully estimating EROEI. But really in both cases, what you need to know is what them major contributors are--it's not really worth tracking down sources that are only going to affect the outcome by a tiny fraction of a percentage.
The most recent estimate for GWP of methane are a bit higher than what you indicate, and of course the rate over the shorter time periods when most of the methane remains as such in the atmosphere are much higher--over 100 times CO2 equivalent, by latest estimates, IIRC.
I think this is the article, but all you get here is the abstract that doesn't have these details.
http://www.haowomen.info/cgi/content/abstract/sci;326/5953/716
I believe I got the figures from a quote someone at realclimate pulled out of this article. I'll see if I can track it down.
{Edit: Aha, I found it
post # 220 at http://www.realclimate.org/?comments_popup=3100
"There is a paper by Shindell et al., “Improved Attribution of Climate Forcing to Emissions“,
http://www.sciencemag.org/cgi/content/abstract/326/5953/716
This paper argues that methane is more potent than previously realised due to the interaction with black carbon. The paper gives a revised Global Warming Potential for methane measured over 100 years as 33. This is an increase of over 30% compared to the value of 21 given in the IPCC Second Assessment Report used for the Kyoto Protocol. Over 20 years. Shindell et al. calculate this GWP to be 105. If this measure were used the climate impact of methane (e.g. for Plan B above), it would be 5 times the value agreed at Kyoto.
This is important in assessing the impact of animal husbandry, particulary for cattle and sheep. Are Shindell et. al. right?
[Response: Of course we are! ;) - gavin]
Comment by Geoff Beacon — 13 March 2010 @ 2:50 PM"
US mines 1 billion tons of coal(2 billion tons of CO2 by combustion) releasing 67.6 million metric tons of methane as CO2 equivalent.
Abandoned coal mines released 5.9 million tons of methane as CO2 equivalent.
Natural gas piping(leaks?) producing 23 Tcf of natural gas releases(1.22 billion tons of CO2 by combustion) 96.4 million tons of CO2.
This sugessts the pipeline leakage is less than you think as 96.4/1220 = 7%/25=0.3% as methane leaks.
About 51% of methane comes from cows, waste treatment and landfills(various forms of crap).
http://epa.gov/methane/sources.html
In addition to the methane emissions from mining, I wonder what the warming contribution from the black soot would do to the value for coal-generated burning. Soot has a much lower half-life, but it is a strong contributor nonetheless.
http://en.wikipedia.org/wiki/Black_carbon#Black_carbon_contribution_to_g...
If this is indeed the case, I believe the argument would shift strongly back in favour of gas.
I suspect that the black carbon also is involved in sea ice and glacier melt. Its residence time on the snow surface I would think is all summer long. In the case of glacier abalation zones, does last years black carbon add to this years, and also last years ...
The total forcing from CH4 is a fraction of the CO2 forcing, so we can't have any really large unknown sources. Also CH4 as a driver I find less scary than CO2. With CO2 by the time it becomes obvious tha GW is getting you, cessation of emissions will still leave you with a problem that will last many centuries. At least with methane, the effects go away after a few decades, so the strategy of BAU until you get slapped hard by mother nature doesn't seem quite so suicidal.
I think methane breakdown in the stratosphere might be an important source of stratospheric water vapor, which has a warming effect. So the indirect effects of methane which come from its effects on atmospheric chemistry are not easy to determine.
Hi Chemist,
B52 planes:
The amount of methane is coal seams depends on the rank of the coal (higher rank. more methane), its porosity and pore size distribution, the depth of the seam (higher pressure, more methane) etc. Of course, we can only measure the adsorption capacity of coals after they have been mined and exposed to air.
Here are some measurements of adsorption capacities of US coals (on a dry, ash-free basis):
- 150-600 scf per ton of bituminous coal (dry and ash free) as the pressure increases from 100-1,000 psi,
- similar values for bituminous coals for the same pressure range,
- up to 100 scf per ton of subbituminous coal (Powder Basin, WY) for the same pressure range,
- up to 800 scf per ton of anthracite.
Exactly the point I was going to make - coal mines leak methane like crazy.
And chris - would you care to comment on the residence time of methane in the atmosphere. How long before it oxidises to CO2?
Its easy to put the fear of God into an ignorant public by claiming methane is even more potent than CO2 and the concentration has risen from 1500 to 1800 parts per billion (?) - i.e. 1.5 to 1.8 parts per million, i.e. from 0.00015 to 0.00018% (hope I got my decimals and all that right).
Absolutely, about CBM. I wish I had a typical/average for CH4 / tonne of coal in a few different regions.
The CDIAC link above says CH4 has a lifetime of around 12 years, and that the concentration has risen from 700 to 1865/1741 (two locations) parts per billion since 1750.
I think the key point is that the impact CH4 has depends on its instantaneous release rate - give or take a decade, whereas for CO2 the cumulative effect is more important.
Related question Chris (and thanks for the article, its a factor I hadn't thought of before).
How does the distribution of the gases in the atmosphere impact the greenhouse effect consequences?
As I remember methane is a very light gas, whereas CO2 is famously heavy. As a consequence the distribution in the atmosphere should vary quite substantially, and with it the compound effect (and probability of dissociation).
CO2, and Methane are both considered to be well mixed. Although scientists are using the slight local variations in CO2 to try to characterise sources and sinks of the gas. Certainly in the troposphere turbulent motion keeps these things well mixed. I guess if you get really high up, like say 20 to 50 kilometers you might start to see some effects from molecular weight. Water vapor, on the other hand is highly variable, as it gets precipitated out by low temperatures.
CO2 is not as prevalent in the stratosphere. That's one reason that jets are so damaging--they inject CO2 into a layer of the atmosphere that otherwise would have very little for a long while. Of course, as concentrations rise in the lower layers, ultimately CO2 levels will also rise in the higher ones.
garyp,
You can get model estimates of the distribution of gases in the atmosphere and spectra of the IR Radiation escaping to space by running the MODTRAN IR atmospheric radiation code at the site Understanding the Forecast.
Lots of fun!
You mean 0.00007 to 0.00019%
:-)
How long does it take CH4 to degrade to H2O and CO2? I seem to recall a figure of four years?? Regardless, over time the effects cannot be considered cumulative but certainly significant on an ongoing basis.
How long does it take for methane to break down?
The CDIAC link above says CH4 has a 12 year lifetime. However, the GWP calculation producing the factor 25x over CO2 is integrated over a common 100 year horizon. Over a shorter period, the GWP is even higher.
I recall a five years half life (that is diferent from a 5 years lifetime). Also, estimates vary between it being 80 to 100 times more powerfull than CO2 before degradation, and I guess the 25 times more powerfull comes from the integral of the greenhouse power through time, taking into account the degradation (that means, with a decreasing exponential power). It surely can't be added up over time.
As the degradation is random, the half life is well defined, not the lifetime. But people have trouble dealing with half lifes, so the press likes to talk about lifetime, and everybody puts their zero at a diferent amount of degradation, so everybody's figure differ and there is too much confusion around simple numbers.
Normally the lifetime is the average langth of time a molecule is expecxted to be around. The half-life is the LOG(2) times the lifetime (.693 last I recall). Lifetime is the more natural concept mathmetically as the density over time goes like exp(-t/L) where L is the "lifetime".
For CO2, throw the exponential decline out the window.
They refer to it as a "residence time" and from what I have studied, it goes like 1/sqrt(time), which is an incredibly fat-tailed effect.
The reason most of the CO skeptic clowns can't figure this out is that fat-tail responses often have a "quick" part and then a slow, long tail.

Well, they see the quick part and immediately extrapolate to the exponential and note that the half-life is just a few years. Wrong. The tail turns out to be 100 years or more. I wrote about it here:
http://mobjectivist.blogspot.com/2010/04/fat-tail-in-co2-persistence.html
Methane has a much shorter residence time -- after all it is encapsulated energy and something will eventually take a bite out of it.
On the other hand, CO2 sits up there in the atmosphere diffusing and dispersing trying to find a home, and it needs just the right conditions to get sequestered out of the atmosphere. My interpretation is that it is diffusion limited over a range of rates. So CO2 is much more "inert" than Methane.
Very nicely put.
The 100 year GWP for methane is mostly the result of averaging the effect over 100 years, even though most of that happens in the first two decades or less.
Do you have similar graphs for methane? My impression is that, because of the reactivity you mention, it has a much less "fat tail."
Leak rates are very interesting, but the timescale of GWP seems to be the wild variable:
From wikipedia; GWP of:
72 over 20 years
25 over 100
7.6 over 500
How to estimate the harm?
I could make the following argument for using the 20 years GWP number of 72:
Given that:
- The chief danger is kicking off feedback effects that could dwarf our human contributions (e.g. tundra and clathrates methane release, sea ice albedo and ice sheet collapse, east-amazon and boreal forest death, etc)
- A couple of decades later, there's ample evidence that these feedbacks are now underway.
It follows that:
- Our goal is to not cross that threshold to runaway warming. Otherwise, all is lost and it doesn't matter.
Thus:
- Short term warming potential is the most critical by far in terms of jump-starting these feedbacks = methane inordinately dangerous.
In my view the real answer is neither, we just can't have any fossil fuels anymore, period.
If by runaway warming you mean a transition to a Venuslike climate, the answer is that there is no danger of that happening. That there might be some climate tipping points which push the system into some new unexpected state, those are always possible. I don't think anyone can say that the likelihood of hitting such a tipping point is any more likely going from 390-400ppm or from say 490-500ppm. We just don't know about these. The more we let things up, the greater the odds, but we don't know where the safe/unsafe level is.
My own research into extremes seems to indicate that falling Oxygen concentrations is the real issue. Low Oxygen is strongly correlated with extinctions. Also high C02 levels seem to precede the oxygen related extinction events.
I'm being very general here but live itself seems sensitive to conditions and the mass of life is so large that mass die offs themselves have a feedback effect.
And article that questions the concept but its a good enough link.
http://www.sciencedaily.com/releases/2008/09/080908073751.htm
Understand a oxygen event need not be long lived just long enough to seriously disrupt the ecosystem then the collapse itself could finish the job. But from everything I've read this seems to be the top smoking gun if you will that I've found.
Is it your own research, or did you just pull stuff from some science news links?
Please go into more detail since it is your own research, and you should be able to defend your hypothesis.
James Hansen in his book is adamant about keeping CO2 below 350ppm.
http://stormsofmygrandchildren.com/
Furthermore, according to him, a Venus-like scenario is all too likely for his comfort (and mine too, I may add).
Yup. Much as I admire enemy, I'll go with Hansen when it comes to judgments about climate and planetary systems.
Keep in mind that methane, large as it is, is not the only re-enforcing feedback mechanism:
The other big one is water vapor, itself a greenhouse gas, and of course on a planet 3/4 covered with water, the more heat, the more water vapor. Yes, some of that will form the kind of clouds that reflect light, but some clouds will also have a larger net effect of keeping heat in. This has its largest effects at night and in winter, which would otherwise get much colder.
Loss of reflectivity or albedo as less and less of the earth and ocean is covered with ice and snow mean more solar energy is absorbed.
Hotter oceans will not only absorb less CO2, but could start off-gassing the CO2 that has already been absorbed.
The fact that the ocean has already absorbed about half the CO2 we have emitted means that it is less able to absorb further CO2.
The warmer and more acidic oceans (especially as they are more exposed to UV from damaged ozone layer) support fewer plankton, important organisms for sequestering CO2 (and for providing about half of the oxygen from photosynthesis).
Soils, as they dry out under increased heat, will emit further huge quantities of CO2.
Forests and other flora will more readily burn as they dry out and heat up, releasing yet more CO2.
Even if they don't burn, heat- and drought-stressed plants won't be as healthy, so won't be able to draw CO2 out of the atmosphere as easily.
As the atmospheric chemistry changes, hydroxyls, crucial in breaking down methane and other GHGs, deplete.
Once all ice is gone from the Arctic Ocean during the summer, the water will no longer be cooled by the ice changing state as it melts and will start to warm far beyond what it has so far.
Human mediated feedbacks include the likely increased use of air conditioning by those who can afford it, AC that is not only mostly powered by CO2 emitting coal, but also uses refrigerants, powerful GHGs, some of which inevitably leaks into the atmosphere.
And there are many others--please add your own; I'm trying to keep track of all of them.
Here's something I found from a TOD posting from Nov 27 '08 6:07 from a thread on phasing out coal (sorry, I didn't record the poster's name):
"you reach the point that water vapour feedback dominates. Essentially, once the oceans reach an average temperature of about 28 Degrees C (about 13 degrees higher than today), you get the situation that evaporation exceeds precipitation in an ongoing cycle (Higher evaporation = higher temperatures = more evaporation, where it cannot rain out fast enough to stop). Very, very quickly (perhaps a century), surface temperatures exceed the boiling point of water; the oceans boil into the atmosphere, increasing the greenhouse effect even more.
Now, of course, the temperature gets so extreme that carbonates start breaking down, so all exposed Carbonates release CO2 back into the atmsophere. Volcanic CO2 will also build up. Even more greenhouse effect.
The huge increase in atmospheric water means that more will br broken down by UV light into oxygen and hydrogen, with the hydrogen escaping. Several hundred million years hence, the water will mostly be gone and the oxygen gradually removed by reaction with lava. We will now have a massive CO2 atmosphere and a searing desert of a surface, much like Venus today...Something to think about next time you start your car up"
Hi enemy,
Good place to start knowing:
http://www.amazon.com/Six-Degrees-Future-Hotter-Planet/dp/1426203853/ref...
As I pointed out above, those Global Warming Potentials for methane are now estimated to be 105 times CO2 over 20 years, 33 over 100 years. And again, almost all of the actual effect happens in those first 20 years. The lower number is just basically the same number averaged across the period of a century.
You should use Mike's nature trick to hide the decline.
You failed to differentiate between three primary means of burning NG to make electricity.
Central Heat and Power (CHP) - Typically burn in a gas turbine, make electricity, use exhaust to heat steam boiler, make more electricity, use "waste" heat to heat buildings or make chilled water via absorption chilling.
Thermodynamic efficiencies of 80% have been reported.
In other CHP, NG is used to help burn garbage or other bio-mass in steam boilers.
Combined cycle - As above but just dump waste heat. Best efficiency is GE's H series, 60% catalog efficiency.
Direct turbine (high 30's% efficiency) or straight steam (about 40% efficiency, now obsolete).
NG used in CHPs is pretty green if the buildings being heated are well insulated, etc.
NG used in straight turbines, not so much.
Best Hopes for CHPs,
Alan
I strongly suspect that NG power is a better target for carbon capture and storage than coal. Since the number of joules per gram of methane is higher, the increase in overal system cost of capture and disposal ought to be more favorable than for coal.
No.
CH4 +2O2 -->CO2 + 2H2O versus C + O2 --> CO2
Coal produces fairly pure CO2.
Natural gas produces almost as much water as CO2 that needs to be separated and removed.
But the real difference is the higher cost per Btu of natural gas over coal.
Also remember that the amount of natural gas reserves in the world is about 1/3 that of coal even though gas consumption is about the same as coal.
Water should be pretty easy to separate out, as it is easily condensed. Separating the CO2, from the nitrogen is the big issue. At least with NG, the amount of CO2 per usable BTU is less, so you can amortize the cost against 2 to 2.5 times the saleable energy.
In IGCC, NGCC gasification systems nitrogen is removed from air pre-combustion so it isn't an issue. With pulverized coal boiler plants, the CO2 is scrubbed from the exhaust gas which is mainly nitrogen. This makes post-combustion CCS very energy intensive and impractical.
A bit besides the point.
The CCS process is about removing CO2, not producing more energy per pound of CO2. Coal is a plentiful fuel, natural gas is not. The cost of coal is less than half the current low price of natural gas.
If you want to burn a fuel that emits little CO2, try trees or hydrogen gas--not that these are abundant.
Then there is also the burning of NG to keep the compressors going that transport the gas over vast distances and keep the system under pressure. No idea how much they consume vs the amount that they can transport... Someone around who has the numbers?
From: "Preliminary Assessment of the Greenhouse Gas Emissions from Natural Gas obtained by Hydraulic Fracturing"
http://www.eeb.cornell.edu/howarth/GHG%20emissions%20from%20Marcellus%20...
"..If we assume a 1.5% leakage rate, this would have a greenhouse gas warming potential equal to 14.8 g C of CO2 per million joules of energy. This would be additive to the emissions during combustion (13.7 g C of CO2 per million joules of energy) and to the emissions associated with obtaining and transporting the natural gas (very roughly estimated above as 4.5 g C of CO2 per million joules of energy)..."
The transport of any fuel requires significant energy. From the source I cited, it looks like about 30% (4.7g out of 13.7) of the available energy in natural gas is consumed in its transport.
In the US there are pretty good numbers for the last 10 years or so. You can find them here at the EIA:
http://www.eia.gov/dnav/ng/ng_cons_sum_dcu_nus_a.htm
Looks like pipelines consume about 2% of the total US natural gas produced. Fractioning the gas to seperate out the propane and ethane etc takes far more, about 5.5% ( total 7.5% Just for comparison total commercial building consumption in the US is 13.7% or right about twice that amount. That is a lot of buildings heated.)
So, for the EROI interested, US natural gas is below 12 to 1 EROI at the end of the distribution network alone and that does not include compressing and decompressing for winter, or paying the energy cost of drilling, the costs of building the pipelines, local distribution, or end use facility (burners) etc.
-Jon
The numbers you are quoting would seem to include leakage, but I would expect they are mostly natural gas intentionally burned as fuel either in the initial processing, or in powering the pipelines.
Excellent article, Chris, just the kind that makes TOD shine. If you update it at some point, please consider adding a rising indicator for coal-mining methane release, similar to the one for natural gas piping.
Methane is also likely to be the gas that will spell our doom. It is now bubbling out of the tundra and starting to bubble out of parts of the Arctic Ocean sea bed. There are thousands of gigatons of it ready to be released from the latter source, much of it at relatively shallow depths under 100 meters so it would not get dissolved in the water much.
Do the math.
At over 100 times the GWP of CO2 over the short term of its life span in the air (10-20 years), ten thousand gigatons (the amount estimated to be just in the East Siberian Arctic seabed) works out to the equivalent of one million gigatons of CO2. We currently emit about 30 gt CO2 per year.
So a .1% release would more than triple the release from all industrial sources.
This year so far, Arctic sea ice is melting faster and is farther along than any other time in recorded history.
The methane that is being released by global warming is a serious concern because it can become part of a positive feedback that moves out of our control no matter how much we cut back of fossil fuels. Perhaps it is already in that mode and nothing we do will change the outcome, only the timing.
I read that the Deepwater Horizon is an unusually high percent (40%) for such a well, but that it is staying for now in the ocean
http://www.palmbeachpost.com/money/gulf-oil-full-of-methane-adding-new-c...
How much can continue to be absorbed by the ocean? What does that do to ocean life? If this spill is not stopped when does this methane start escaping into the atmosphere and how much will that move up the global warming timeline?
I suppose to some degree those are unknowns as we are preforming what well may be a once in a civilization (or species) experiment.
Phytoplankton not only are a food source for other ocean going species but also provide 1/2 the planet's O2. Will the methane kill phytoplankton or be good for them?
http://news.nationalgeographic.com/news/2004/06/0607_040607_phytoplankton.html
What in BAU is worth the risks we are taking?
Here's my source for the updated global warming potential of methane:
Post #220 at http://www.realclimate.org/?comments_popup=3100
"There is a paper by Shindell et al., “Improved Attribution of Climate Forcing to Emissions“,
http://www.sciencemag.org/cgi/content/abstract/326/5953/716
This paper argues that methane is more potent than previously realised due to the interaction with black carbon. The paper gives a revised Global Warming Potential for methane measured over 100 years as 33. This is an increase of over 30% compared to the value of 21 given in the IPCC Second Assessment Report used for the Kyoto Protocol. Over 20 years. Shindell et al. calculate this GWP to be 105. If this measure were used the climate impact of methane (e.g. for Plan B above), it would be 5 times the value agreed at Kyoto.
This is important in assessing the impact of animal husbandry, particulary for cattle and sheep. Are Shindell et. al. right?
[Response: Of course we are! ;) - gavin]
Comment by Geoff Beacon — 13 March 2010 @ 2:50 PM"
In other words, game over?
Draw your own conclusions.
It's pretty hard to say what the timeline will be for any of this. But so far in crucial areas like Arctic Sea ice melt, things are moving far faster than models from just a few years ago were predicting.
The record rate of melt and record low coverage for this time of year in the Arctic does not bode well. But so far, the fastest retreat has been in the Hudson Bay, far from the huge reserves of methane and clathrate that are said to be under the East Siberian Arctic Sea. But all the areas that have started to melt are doing so earlier and at somewhat faster rates than earlier averages. ESAS is just starting its seasonal melt now. This should be the headlines followed every day, but it is not mentioned in any MSM pretty much anywhere--certainly not as the front-page story it deserves to be.
Would another Paleocene-Eocene Thermal Maximum be "game over"? It would certainly be a huge mess and likely the end of this civilization, but lots of adaptable things would make it through just like the last time.
Where did you get 0.1%? If you have pulled that out of your hat, I can do that with equal validity - as higher temps are inimical to the continued storage of that arctic methane, lets make the assumption that a few percentage points escape, a run away feedback begins, and 0.1% ultimately is what remains in storage rather than what is released. What is the limit of global warming in that scenario?
We explore space to gain insight here on Earth. Apparently Mars had an ocean over up to 1/3 of its surface for a very long time, and was much warmer. Its stable climate system fundamentally changed for an unknown reason. There is no law that I am aware of that a system will remain stable only because it always has been, after all, every person that has experienced a fatal event has a perfect record of avoiding such events prior to that.
"Where did you get 0.1%?"
Ummm, here I thought I had made this too obvious after asking people to do their own math. Unless I am missing something, .1% of one million gts is 100 gts, some three times our current annual industrial emissions of CO2. What is unclear about that?
As to the rest of your post, I have no idea what your are trying to get at. Yes, the earth may have gone into some kind of unstable shift all on its own. But it just didn't this time. We took a world that was well suited to our civilization and pushed it/continue to push it into a very different state.
As Hansen suggests, Venus may be the better planetary comparison here. And something like that is presumably the limit you were wondering about.
So you tell us, what do you think is the limit?
.1% of one million gts is 100 gts, some three times our current annual industrial emissions of CO2. What is unclear about that?
Perfectly clear. What wasn't clear to me was if you had a source for 0.1%. I hoped you had. My choice of words was unfortunate, no confrontation or disagreement intended. The subject matter of feedback amplification is unpleasant. I think you chose 0.1% as a "for example", unsure though. Do you find 1%, 10%, even approaching 100% release plausible? I do. What is the limit? It is plausible that it has already been exceeded, we just don't know. We have difficulty seeing the point of shift to an alternative stable state.
Venus and Mars both appear to have experienced a shift to an alternative stable state in their climate systems. They both are useful models for comparison to Earth's climate system.
"I think you chose 0.1% as a "for example", unsure though."
Yes, sorry, it was a random "for example." Even that minute amount would have that effect.
And yes, I think more is possible. It's just that many people claim that it would not escape all at once so it wouldn't cause a major, sudden jump in atmospheric levels because of methane's short life span in the atmosphere. But only a very tiny fraction of a percent needs to escape to have a huge effects.
And, yes, that would almost surely feed back on itself (and contribute to all the other feed backs), yielding runaway global warming.
One (kind of) limiting factor is that it takes doublings of concentration to get the same level of increase (3 degrees centigrade is the usual quantity I've heard for doubling of concentration of CO2). So on the one hand it takes more and more GHG to increase the next 3 degrees. On the other hand there seem to be plenty of "positive," re-enforcing feed backs available to supply such increases, and very few and very slow "negative," limiting feedbacks (decay of mountains...).
I only looked at electricity production (UK centric so combined cycle power stations) but the same issue applies to using natural gas for cars. The 'benchmark' CO2 emissions for oil are lower than coal, and I'm sure with millions of natural gas cars filling up, leak rates would be higher than for gas power stations. Natural gas powered cars have a higher global warming potential than oil powered vehicles?
Natural gas is the least offensive fossil fuel, but its supply is still finite and extraction can cause problems as well. With the oil disaster in the Gulf of Mexico, some people are once again calling for increased production of biofuels—ethanol and biodiesel—and accelerated research into clean coal. Ignoring the fact that biofuels take as much energy to produce as they provide and that they are only competitive with heavy government subsidies, biofuel boosters are again trying to sell their snake-oil to the public. But the single most damning aspect of biofuel production is the exorbitant amount of water it takes to cook-up a gallon of the stuff. And as it turns out, clean coal is just as bad or worse. The future of energy is more complicated than most people realize.
see http://theresilientearth.com/?q=content/clean-coal-biofuels-will-cause-w...
Natural gas still promotes global warming. Better to switch to nuclear. perhaps a Liquid fluoride thorium reactor which has potential to be cheaper then coal.
The article makes some valid points regarding use of methane in the UK.
The same is not true for the US in comparing coal to methane for effect on climate change because of these reasons:
1. Most new gas power plants are using the combined cycle design which is 60% efficient versus 39% efficiency for coal plants that can be financed in todays power market.
2. The upstream energy costs for coal is huge in the US. Even in the east much coal from Wyoming and Montana is burned because of its low sulphur content. Moving this coal by rail (train runs empty on return) a distance of 2000 miles (3230km) involves an energy cost that reduces by 10% to 20% the net energy content.
3. Most gas pipelines are moving methane that is odorized at the compressor stations, long before it gets near points of use. Detection of leaks is very important to the transmission industry so the amount being leaked compared to that used is extremely small, IMO.
One other comment about the effect of methane (CH4) in the atmosphere. It does eventually oxidize into H2O and CO2 when exposed to sunlight. Thus it will not be accumulating at the same rate as it is produced from leaks in pipes and from coal mines.
The 60%/39% gas/coal difference is similar in the UK and included in the 360 and 890 g CO2/kWh figure I used. Do you think things have improved significantly in the US since that EPA report and it's 1.4% figure? I guess my main concern is whether the leakage rate Europe is experiencing will increase significantly or not as we import a growing amount from Russia and beyond.
Moving this coal by rail (train runs empty on return) a distance of 2000 miles (3230km) involves an energy cost that reduces by 10% to 20% the net energy content.
Could you provide your calculations? This looks too high to me.
Assuming 500 ton-miles per gallon of diesel, the out-trip would account for 4 gallons of fuel per ton. A gallon of diesel is about 140,000 BTU, for about 0.5 million BTU total.
The lowest figure for Powder River Basin coal which pops up in a quick search is 18.8 million BTU per ton. The correct figure appears to be closer to 3% than 20%.
And, as you know, you're being conservative. For example, 500 ton-miles per gallon of diesel is an average for all freight: the figure for coal trains would be somewhat higher.
Anyone have any links to studies relating to NG compressor stations/storage facilities? 'Gasland' seemed to indicate that these are not as benign as we have been led to believe.
Also, in reference to the documentary, in a study which I believe was from Texas A&M, concluded that emissions from NG wells, compressor stations etc.. in and around the Forth Worth area contributed just as much emissions into the atmosphere (daily) as emissions from cars and trucks in the Metro-Area (daily). That would seem pretty damning if it was verified. Anyone have any independent backup?
I haven't had an opportunity to see Gasland yet, but I have read a lot of local news articles on the state of hydrofracking in the Marcellus Shale formation. (NY/PA in the United States)
In PA they've had quite a few blowouts and quite a few spills of residual fracking fluid. There also seems to be evidence that wells are suffering underground blowouts into drinking water aquifers - the aquifer in Dimock, PA became undrinkable within a few years of the beginning of drilling operations.
Natural gas is looking less and less green as time goes by, due to the difficulty of extracting it without side effects.
Anyone who missed Gasland Monday night on HBO might want to watch the PBS interview with filmmaker Josh Fox. It aired back in March.
Just FYI (in case you ever have to argue with a denialist about it): methane is stronger greenhouse gas because it has more atoms in the molecule. The greenhouse effect is caused by infrared absorption, and molecules with more atoms in them absorb more infrared energy. IR absorption is done by the electrons that bond between atoms in a molecule and the way those atoms vibrate. More bonds = more vibrations = more IR absorption.
Diatomic molecules, like 02 and N2 which mostly make up our atmosphere, essentially absorb no IR. CO2 (2 bonds) absorbs some, but it is the next most abundant gas so its effect is significant. Water Vapor is also a significant greenhouse gas, but obviously it is already built into the system its effects tend to be localized and if the concentration gets high enough or the air gets cold enough it condenses back out. There is a potential feedback loop from a hotter atmosphere holding additional water and so getting even hotter. Methane (4 bonds, and lots more vibrational modes for those bonds) is a very strong absorber, but does oxidize to C02 over time. CO2 is chemically inert in the atmosphere, and only gets pulled out by photosynthesis (plants use sunlight to convert CO2 to sugars).
As hydrocarbons get longer they absorb more and more IR, but none are as prevalent as methane. Propane is a worse greenhouse gas than methane but there isn't very much of it getting into the atmosphere.
Nice :)
Things are a bit more complicated.
The big difference in the GHG potential of CO2 and methane comes from the phenomenon of band saturation.
The more CO2 we have in the atmosphere, the smaller will be the effect of adding a certain extra amount of it. In other words, 10 ppm of additional CO2 will block a lot more of the outgoing IR energy flux if they were added to an atmosphere containing 100 ppm of CO2, than if they are added to an atmosphere containing 385 ppm CO2. In fact, the amount of outgoing IR radiation "blocked" by the extra CO2 goes down in some exponential fashion as the total CO2 concentration increases.
Currently, the methane adsorption band is far from the saturation point (too little methane in the atmosphere, about 1.8 ppm). This makes every molecule of methane added to the atmosphere at this point about 21 times more powerful in blocking outgoing IR radiation than a molecule of added CO2.
Technically correct but over-simplified. As CO2 concentrations increase, the CO2 absorbtion band becomes saturated BUT a) that absorbtion band wavelength for CO2 changes with altitude b) a thinner layer of atmosphere becomes opaque to IR at the CO2 wavelength, leaving more of the atmosphere below re-radiating its IR back to earth or to other atmospheric molecules.
So a primary effect of increasing CO2 even beyond present levels is to push higher the altitude at which earth can not release any radiation energy to space, and to push further toward the poles and into other very dry regions where water vapour doesn't dominate, the effects of CO2's increased IR blockage. Still a net increase in global temperature, all carefully (and very conservatively) included already in IPCC's conclusions.
Rather than attempting to do the science ourselves based on hearsay and questionable sources, we should simply accept the results of the IPCC's very thorough reports. No evidence to date causes any of their reports or publications to be determined incorrect.
What's wrong with doing the science here?
Do you know how hard it is to dig up that nice piece of explanation you just gave?
Ok, fine, but I'm just an amateur observer of climate science, and shouldn't be taken over a qualified expert such as all those phd's who contributed to the IPCC reports, in case there's any discrepancy. Personally, I suspect that if anything, the IPCC reports present a "best case" scenario, since a LOT of powerfull political reviewers had IMHO far too much power to influence the wording and details of the outcomes (anything not absolutely proven must be removed etc. etc., leaving a lot of "90% sure" stuff out.)
Exactly right man; best case scenario.
...After whatever lobby groups and special interests and oil producing countries go through it and protest this phrasing or that wording. Pick up a book by a climate scientist (e.g. James Hansen) and you'll get a whiff of how panicky they really are, and what some of the horror effects are.
I'm also amazed how separate the Peak Oil and Global Warming camps are - these are both just effects of our fossil use, and both well supported by fact - so you'd think they'd both appeal to us fact-based, system-thinker types, No?!? UNITE!
Couldn't agree more. The scary thing about peak oil is that big business will turn to coal for F-T conversion to methane and liquid fuels. Even more CO2 per unit of work. The Chinese are going big with conversion of coal to DME. Coal was the first fossil fuel burned and will be the last fossil fuel burned. The whole world is riding the climate change roller coaster and no one can get off.
Nice. Methane also "plugs" a different "hole" in the spectrum that isn't blocked by other gasses, IIRC.
Maybe lengould could be a lamb and find these spectrum charts for us lazy bones?
What matters are the spectral positions of the lines, and the line shapes. IIRC the CO2 lines fall off exponentially with distance from the line center. So in order to increase the amount of spectrum blocked by a given absorption line you got to increase the concentration exponentially. The inverse of an exponential is a logarithm, which is why for GHG with saturated lines the effect depends upon the logarithm of the concentration. I think CH4 behaves similarly, but the constant in from of the logarithm is smaller. Some of the other trace GHGes supposedly have a square root depenedency, not sure where that comes from.
In any case simple explanations, like changing the height where things become transparent don't describe things very well, as you have a spectrum of frequencies, and of atmospheric levels. In some windows most IR photons can directly escape, in others they don't get very far. Then you have intermediate regions (of spectrum). So you probably got to write/run a detailed radiative transport code to get the true picture.
Len,
I was only trying to make a point about band saturation in order to explain the differences in GHG potential between CO2 and CH4. By definition, any "explanation" that does not include a detailed, layer-by-layer analysis of IR radiation fluxes through the atmosphere will be oversimplified. Only such an analysis can account for gas interactions, the differences in gas composition as we move from the troposphere to the stratosphere, or from the equator to the poles, etc.
The IPCC models account for all these factors and we should all believe their quantitative conclusions. But, we can still come up with qualitative explanations for key phenomena that are based on solid science and are simple enough for most of us to understand.
When methane breaks down in the atmosphere, does it release heat?
Can the heat from the reaction contribute to global warming?
(I don't have much technical know how, so forgive me if this is being discussed in this thread)
It does, but there isn't that much methane for the heat to be significant. Not compared to capturing extra heat every hour of every day. Even though the amounts are very small, it is always working.
And I was simplifying to make it simple, lol. You can always get more complicated. Its good to have it get more complicated in subsequent posts.
Thks-simplified and understandable..
http://www.breitbart.tv/surf-at-pensacola-beach-boiling-like-acid/
Anybody know what this is?
Thank you!
I have no idea what the cause of the bubbles might be but my first guess would be that some water saturated with methane (at some significant depth) has upwelled at the waterfront and the methane is bubbling out due to the lower pressure at the surface.
I have seen methane bubble up fast enough in a stagnant pond to ignite it with a match.
Maybe there is a new dead zone in the immediate nieghborhood as the result of a plume of oily water passing by.Somebody here should know if a dead zone could produce methane in large enough quantities over short period of time capable of producing such bubbles.
That might be it. But given the huge quantities of methane being released from the well head, this could be methane that remained dissolved in a large hunk of oil that stayed together all the way to the beach. As you say, when it comes to the relative warmth and low pressure of the surface, it finally is dissociating.
But who knows.
I heard this morning that there was a huge oil slick that washed up near Pensacola over night.
This is getting weirder and uglier by the day.
There was some discussion of this possibility just recently on one of the Environmental threads over at Peak Oil Forums. Weird to see this happen right after this discussion.
One of our new too-prosperous nieghbors from Florida is up to spend the next few weeks in the mountians and says that waterfront hotel rates are about half what they usually are at this time of year where he lives.
"That" is a really low-res video, so who can tell? I tried it in HD, it still looked all blocky. Ticks me off, I cannot tell if some guy is just now noticing something that is rare-but-natural, or if it is truly unprecedented.
An issue with Natural Gas production which I've often raised with zero response is the amount of CO2 which may come out of the ground along with the natural gas. eg. IAEA - Notes on HTR applications in Methanol Production discusses "how should the producing company deal with the 71% CO2 which comes out of the well along with the Natural Gas?" This paper considers some other options than simply releasing it, but I've seen Japanese papers discussing how cost-effective their separation-and-release technology is.
Perhaps Indonesian Natural Gas reservoirs are uniquely high in CO2 content? I've never seen the question discussed anywhere else....?
I think the separation of CO2 plus methane is fairly straightforward. The better NG drilling outfits will seperate it out and reinject it. I have seen NG companies run adds that say they are capturing and sequestering CO2. True, but deceptive, as the general public probably gets the impression that all the carbon is sequestered, not just the associated CO2.
1) Ok, so if "the better outfits" re-inject it, why doesn't everybody? Is it not a regulatory requirement? Why not?
2) How about foreign, esp. Algerian and Russian sources of imports?
I'd also be interested in how much reservoir CO2 can a cu ft of delivered natural gas contain and still meet the ?1,000 btu per cu ft? (i think, or whatever) standard required for commercial gas?
Can you give some examples? I am only aware of the Sleipner project where CO2 is separated from the natural gas produced and re-injected it into a saline aquifer, located above the gas field.
Nobody has mentioned the boiling off of LNG by LNG tankers to keep the LNG cold. Some vessels use it for fuel. How many don't? If they don't, do they flare it or just vent it? Is this important?
Significant energy is used to cool LNG and then regasify it at the point of delivery. Increases the carbon-intensity of LNG relative to "local" methane.
For sure. Importing LNG is not really a "green" solution.
That colour,green,is getting painted on some horrible dogs these days and NG is no exception.In Queensland,Australia,where there are huge coal reserves,the Coal Seam Gas industry is up and running.
Some of the extraction is taking place in high value agricultural land and farmers are getting very pinged off with roads,pipelines and well heads all over their paddocks.Hyper saline water in large quantities is a byproduct of the extraction process.Up to date the gas companies have been storing it in evaporation ponds,unsealed.That is hardly a sustainable process as it is going to leave a lot of salt lying around or saline water getting into the water table.Desalination is a solution but that will be very expensive in terms of energy and infrastructure.
This gas is being produced for export and the companies concerned are interested in a quick buck regardless of the damage they cause.The state government is responsible for regulation but they have a good track record as boosters for industry. Not so much of a reputation for long term vision.
NG is probably a tad less damaging than extracting and burning coal but both fuels need to be relegated to history as soon as possible.
Here's to a nuclear future.
In some Australian mines it has been said fugitive methane is equivalent to about 300 kg of CO2 for every tonne of coal mined. I infer that is 300/25 = 12 kg of methane. Of course we could reduce methane in agriculture by eating less meat. Cows, sheep and I presume chickens all produce the stuff. A lower meat diet could help both human and planetary health.
The issue of fugitive methane needs to be sorted out to pre-empt critics of the Methane Economy. As it I see it the advantages are
1) multiple sources of methane
Biogas, nat gas, coal seam gas, converted syngas, synthetic methane perhaps made with nuclear hydrogen can all be blended.
2) sunk costs in applications
The gas grid, 8 million NGVs worldwide, gas fired peaking plant to cover lulls in wind generation.
Some kind of standard will need to be policed at point source leakages with a roving team of inspectors.
This may be off-topic and if so I apologize. I am new here. I am a very scared Gulf Coast resident who has been trying to learn all I can to mitigate my fear. Unfortunately, in my "research" I have run across sites that posit a doomsday scenario, namely - the "methane tsunami," resulting from pressurized methane cracking the formation and the seabed. I know there are knowledgable, credible engineers and geologists on this board. Please tell me: Is this possible? Is it probable? What -- if any -- would be the warning signs? Thank you in advance for indulging a first-time commenter.
Welcome to TOD!
I'm not a geologist nor am I an engineer, but I do keep a list of "Top 100 Worries." The methane tsunami is not on that list, which I've made up largely by reading and studying links on TOD during the past four years.
You should have a geologist answer your question, however. We have some good petroleum geologists who make comments on TOD; I leave it to you to find out who they are.
Many thanks, Don
I feel you: I live in New Orleans.
I understood that, under those pressures and temperatures, methane forms ice: hence the problem they had with the first big cap they tried to put over the well: the methane ice crystals that built up and clogged the thing, and why they're pumping warm methanol into the new cap. The only reason why natural gas is coming up from the reservoir is because of the high temperatures several miles below the sea-floor. I guess the bid question for me would be that if this were a likely scenario, and I'm not convinced it is, why hasn't it happened yet? The reservoir was compromised the minute they punched a hole in it.
But consider this: the pressure in the reservoir is slowly being reduced by the "production" associated with the leak and we know from prior experience that a reservoir's pressure will often need to be supplemented within a few years of production just to keep its rate of extraction constant. In Norwegian deep water rigs they often use carbon dioxide to artificially pressurize the reservoir and sustain production.
Basically, everyday the pressure in that reservoir is being reduced. At what rate it's impossible to say for certain, but we know that it is.
Thanks, Dan. I'm in New Orleans, too -- not far inland enough to be comfortable, you know what I'm saying? But I guess I can take some solace from your answer. I appreciate it.
Tsunamis are created with a minority of undersea earthquakes, most undersea earthquakes do NOT produce tsunamis.
A LARGE amount of water is displaced by a sudden (within seconds) shift of seafloor.
The reservoir is 13,000' below the seafloor. The oil & gas solution is soaked in sand (think wet beach 60' thick, but wet with oil & gas). The oil & gas mixture has to travel through sand out of the formation and then, somehow, up 2.5 miles to the bottom of the seafloor.
The gas does come out from the oil @ 5,000' down, but it is compressed significantly (over 2,000 psi) and begins to freeze immediately (into methane ice). So the volume of gas is MUCH smaller than at sea level. And the volume of water displaced is to.
We humans provided a hole, perhaps close to a foot diameter, down those 13,000' and lined it with steel. For more oil to come out the hole has to be enlarged (bit by bit), the oil & gas has to come out of the sand it has spent the last xx million years in, etc.
WORST WORST WORST case, all of that oil and gas would come out in months, not in seconds. No tsunami.
Hope that helps :-)
-------
If a tsunami hits a sandy beach, it can roll up and wipe out fishing villages & bottom floors of resort hotels. If they hit a swamp, not too much bad happens. Water goes up, covers marsh grass and maybe cypress trees, and then goes down. Not good for nesting birds and their eggs, but otherwise I cannot see much impact.
Alan in Lower Garden
Nat gas ain't green. It's infrared.
Note that I don't know anything about coal mining, but I hear that:
With respect to coal-mine-methane, right now there are two incentives operating: 1)Keep gas-air mixtures below 1% in the mine to prevent explosion, 2)Do this for the least net cost thru a combination of drainage (pre-collection) and dilution by ventilation air. Pre-collection allows sale of the gas and reduction in energy intensive ventilation but requires additional equipment/efforts.
It seems that the potential to shift the mix to lower release levels by incentivizing collection, exists (perhaps a GHG tax?).
Another mechanism for reducing emissions would be to use the ventilation air for combustion air in on-site generation. I did an undergrad paper in 1997 on the potential for this (as a power BSEE student).
Here's a more professional effort by someone more competent:
http://www.smenet.org/uvc/mineventpapers/pdf/045.pdf
Chris: see: Lilieveld, J, S. Lechtenboehmer, S.S. Assonov, C.A.M. Breeninkmeijer, C. Dient, M. Fischedick, & T. Hanke (2005) "Low methane leakage from gas pipelines," Nature, vol. 434:841-842. global gas production of ~2,600 Bcm/yr; tested leakage within Russian pipelines at ~0.7% pa (0.4-1.6%), and Russian gas transport systems overall (incl at wells) 1.4% (1.0-2.5%), "comparable to the amount lost from pipelines in the U.S. (1.5+/-0.5%). Lilieveld is (was?) with Max Planck Institut. Another paper, dated and peripheral: Tohjima, Y., S. Maksyutov, T. Machida, G. Inoue (1996) "Airborne measurements of atmospheric methane over oil fields in western Siberia," Geophys Res Letts, vol 23:13:1621-1624.
I calculate a methane leakage rate for US coal-fired electricity: (EIA, 2007) coal CH4 emissions of 2.84 million tonnes, 1,998 billion kWh net generation from coal, 92.6 percent of U.S. coal to power plants, thus 1.32 g CH4/kWh. Or, if we use CH4 as 25xCO2, 32.9 g CO2e/kWh. The coal emission rate includes abandoned mines, and (of course) mixes high rates from subsurface and generally lower rates from surface mines. A similar calculation for natural gas generation yields 3.11 g CH4/kWh and 77.6 g CO2e/kWh, also highly variable.
Furthermore, the "ancillary" burden of LNG, if one follows the CO2 and CH4 sources from field to liquefaction to LNG carriers to terminal is 38 percent above combustion alone, according to a study I did on the Australia to Southern California LNG supply chain (Cabrillo Deepwater Port, Climate Mitigation Services, 2006). Feed LNG to a power plant and we'd have a problem similar to your 1 percent methane leakage rate.